
Therapeutic implications for ovarian cancer emerging from the Tumor Cancer Genome Atlas
Introduction
Today, all epithelial ovarian cancers (EOC) are treated with the same approach which involves a debulking surgery and chemotherapy with a carboplatin doublet, usually paclitaxel, for six cycles. These cycles of chemotherapy can be administered in a neoadjuvant, sandwiched, or adjuvant fashion. The outcome is the same and depends on the quality of the debulking and the responsiveness to platinum. Despite an 80% response rate, less than 20% of women will be cured, and most will eventually recur. Patients with recurrent disease are usually incurable. Five year survival for stage III ovarian cancer, the stage at which EOC is commonly diagnosed, is between 35% and 45%. The current combined surgical-chemotherapy approach has reached a plateau of efficacy. Cells protect themselves from environmental and physiological pressures through complex adaptation strategies including cell cycle checkpoints, DNA damage response pathways, programmed cell death (1) and other mechanisms. When imbalance between DNA damage/repair and activation/inactivation occur in these processes, through carcinogenesis or other intrinsic or extrinsic anomalies, cells might become cancerous. These complex adaptation pathways are being discovered through constant molecular biology discoveries, although our understanding remains limited. The TCGA data helps make further inroads in our understanding of the biology of high grade papillary serous ovarian cancer (HGSOC).
In the last decade, an effort to better understand the biology of ovarian cancer led to its inclusion in The Cancer Genome Atlas (TCGA) project (2). The analysis included 489 high grade ovarian papillary cystadenocarcinomas or papillary serous ovarian cancers (HGSOC) of individual patients. The TCGA project produced the following results:
- It confirmed that mutations affecting p53 expression are present in most HGSOC (96%). The TP53 gene encodes a tumor suppressor protein. The p53 protein is the guardian of the genome of normal cells. It acts by arresting growth, holding the cell cycle at the G1/S regulation checkpoint on DNA damage recognition, activating DNA repair proteins when DNA has sustained damaged, and initiating apoptosis when DNA damage is beyond repair;
- The gene expression patterns of HGSOC could be classified in signatures that correlate with poor or better survival;
- Similarly, four distinct subtypes of ovarian cancer could be determined through examination of RNA transcription and DNA methylation patterns;
- Mutations of BRCA1 and BRCA2 genes were found in 20% of cases and methylation-mediated loss of expression in 11%;
- Genes expressed in various targetable pathways were matched with existing Food and Drug Administration (FDA) approved or experimental therapeutic agents.
This review will attempt to associate the TCGA discoveries and other genomic analyses of ovarian cancer with potential treatment approaches for HGSOC. Table 1 describes therapeutic targets that have been identified, and selected ones will be further described in ensuing sections (for more details consult: http://cancergenome.nih.gov/newsevents/newsannouncements/ovarianpaper and https://icgc.org/).
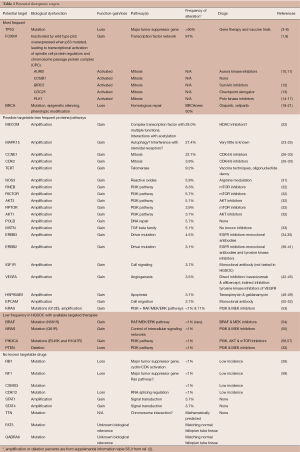
Full table
TP53, the guardian of cellular homeostasis
The TP53 tumor suppressor gene which encodes p53 has long been recognized as a critical regulator of cell proliferation and as a frequent target for mutation in cancer (59). The TCGA project identified TP53 mutations in 96% of ovarian cancers (2). The p53 protein binds to DNA and to a rich network of proteins that are involved in response to DNA damage and other cellular stresses, DNA repair, and cell growth (60). Many of the proteins that either interact with p53 or are part of p53-regulated pathways have been related to ovarian cancer and are discussed below as potential therapeutic targets (e.g., BRCA1, BRCA2, the FoxM1 network).
The most intensely studied function of the p53 protein is as a transcriptional activator. The p53 protein consists of an N-terminal trans-activating domain, a central DNA binding region, and a C-terminal oligomerization domain. In non-stressed conditions, the p53 protein is associated with the MDM2 and MDM4 proteins, which promote the ubiquitination and rapid degradation of p53 (61). A tightly regulated negative feedback loop controls p53 levels, which are typically low. In response to DNA damage and other cellular stressors, these regulatory proteins are inhibited by a variety of upstream proteins, and p53 is released. The p53 protein tetramers bind to DNA and activate the transcription of a large network of genes involved with DNA repair, cellular growth arrest, and apoptosis. The p53 protein acts as a cell cycle checkpoint regulator because it becomes active in cells that exhibit damaged DNA. The p53-mediated induction of several cell cycle regulating genes such as p21 prevents cells from entering G1 or continuing past the G2/M cell cycle checkpoint. There is clear evidence that p53 is also involved in regulating expression of genes that promote apoptosis through both intrinsic (Fas) and extrinsic pathways (Bax). Because of these checkpoint and apoptotic functions, the TP53 gene has been labeled the “guardian of genome”. If there is loss of p53 function due to mutation, deletion, or down-regulation, then cells proceed though the cell cycle despite DNA damage and are prone to further mutations, including potentially oncogenic changes (60).
In addition to its established role as a tumor suppressor through its regulation of gene transcription, several transcription-independent p53 functions have emerged in recent years. These functions include regulation of microRNA networks, effects on mitochondrial survival proteins, and possibly direct involvement of p53 in DNA repair pathways (60,62). Although the network of p53 interactions is very complex, the variety of protein targets with diverse mechanisms within these pathways could provide attractive targets for interventions that bypass specific mutational defects in p53 function.
The analysis of TP53 mutations in databases from cancer cell lines, animal models of cancer and the TCGA human cancer database has yielded many insights into TP53 biology that complement experimental studies. Early on, TP53 was found to be the most commonly somatically mutated tumor suppressor gene in human cancers (59). The database of somatic TP53 mutations found in tumors now exceeds 45,000 entries (63). Additionally, inherited germ line TP53 mutations were found to be responsible for Li-Fraumeni syndrome, a cancer predisposition syndrome characterized by a number of early onset cancers, most notably breast and sarcomas, but not HGSOC germline ovarian cancer type (64). These specific differences in cancer types allude to the complex biology of TP53, where various mutations affect the host in different ways.
Most TP53 alterations are missense point mutations, but nonsense point mutations, frameshift alterations, and large deletions also occur. TP53 point mutations cluster in the central DNA binding domain. The two main molecular mechanisms that abrogate wild type p53 activity are modification of the folded conformation of the DNA binding domain or direct alteration of amino acids that contact DNA (59). The result is that p53 protein oligomers fail to bind DNA and cannot exert their growth inhibitory effects. Many of these mutations are “dominant negative”, such that the presence of the mutation suppresses the activity of residual wild type p53. Some mutations gain growth stimulatory or “oncogenic” properties, thought to be due to an inhibition of mutant p53 on the homologous p53 family members p73 and p63, thereby reducing their transcriptional activity (60,65).
Several strategies have attempted to exploit the p53 pathways in targeted therapeutics. Small molecule screens have produced compounds that bind to either full-length p53 or the core DNA-binding domain of mutant p53 to restore its normal activity. In another approach, molecules that inhibit the protein-protein interaction of p53 with MDM2 have been developed that show clear anti-tumor activity in preclinical animal models. Also, molecules and drug combinations have been tested that selectively kill tumor cells by activating mutant or wild-type p53. Recombinant adenovirus-based gene therapy and anti-p53 vaccines are another method that have been attempted to achieve tumor regression through the p53 pathway (60). The new insights gained from the TCGA project will provide new strategies to more accurately target proteins associated with specific p53 defects in ovarian cancer that can be identified during diagnostic testing.
Genetic & epigenetic profiles and therapies
Efforts associated with TCGA have greatly expanded our knowledge of the genetic and epigenetic (specifically, DNA methylation) landscape of HGSOC (2).
Epigenetic studies of HGSOC
Analysis of epigenetic silencing, through correlation of patterns of DNA methylation and reduced gene expression, also revealed four distinct subtypes (2). Unlike the gene expression subtypes, these epigenetic clusters did show significant correlation with survival and other metrics (age, functional BRCA inactivation). Unlike the expression subtypes, the epigenetic clusters were only modestly ‘stable’, insofar as that application of their signatures to independent datasets did not always fully or faithfully recapitulate the subtypes. Finally, a similar clustering analysis was performed on the expression of micro-RNAs (miRNAs), small (~22 nucleotides) non-coding RNA molecules that function in RNA silencing and other aspects of post-transcriptional regulation of gene expression, and generated three distinct subtypes. Interestingly, two of the miRNA clusters overlapped significantly with two of the mRNA subtypes, while one of these miRNA subtypes was associated with significantly longer survival than the other two. These clustering analyses not only provide an unexpected level of stratification and cohesion within the spectrum of HGSOCs, but also provide an enduring resource that can be mined to significant scientific and potentially, clinical effects.
In addition to the analysis of expression of coding RNAs, utilization of miRNA profiles may prove imminently useful as a screening tool. An increasing number of cancer-associated miRNAs (‘oncomirs’) have been implicated in every step of pathogenesis, from initiation to metastasis to drug resistance. Recently, miR-152 and miR-185 have been found to be down-regulated in platinum-resistant HGSOC (66-68) and up-regulating them promotes platinum sensitivity (68). Conversely, other miRNAs (e.g., miR-93, miR-182, miR-199, miR-214) appear to be specifically up-regulated in or to promote resistant disease [myriad references, searchable at the miRCancer or OncomiRDB database (69,70)].
The molecular mechanisms and pathways connecting miRNAs to metastatic potential and drug resistance are, at best, quite complex and, at worst, wholly unknown. Nonetheless, the strong correlations between some miRNAs and clinical outcomes stand well-poised to be developed into a useful screening target for informing treatment decisions during management of HGSOC. Of particular interest, here, is the recent demonstration that some oncomirs—miR-152 and miR-185—can suppress DNA methyltransferase-1 (DNMT1) and promote DNA hypomethylation (68), thus directly linking genetic and epigenetic regulatory mechanisms. This latter report’s demonstrates a causal relationship between miRNA-regulation of DNMT1. Platinum sensitivity is restored by epigenetic modulators (e.g., DNMT1 inhibitors) (71). A number of HGSOC trials utilizing agents that block DNA hypermethylation are in progress—with varying levels of success (Table 2). With further refinement, genetic sub-typing of HGSOC may be used to stratify patients into groups most likely to benefit from regimens that include epigenetic modifying agents that are either FDA approved [e.g., azacitidine (72,73) and decitabine (74,75)] or under active development [e.g., zebularine (76) and others (77)]. Of particular recent interest here is the possible synergy between epigenetic modifier therapy and vaccination against ovarian cancer antigens. The cancer-testis/cancer-germline antigen NY-ESO-1 is a vaccine target regulated by DNA methylation, Inhibition of DNMT by decitabine augments NY-ESO-1 vaccine therapy, with increased NY-ESO-1 serum antibodies and T cell responses observed in the majority of patients. Antibody spreading to additional tumor antigens was also observed (78).
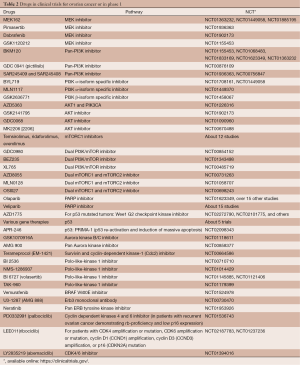
Full table
Expression patterns in HGSOC
The largest and most recent effort selected approximately 1,500 intrinsically variable genes (out of nearly 12,000 genes available across three commercial expression analysis platforms) (2). After characterizing their distinct transcriptional profiles, they identified four mRNA expression ‘clusters’ or subtypes of HGSOC: differentiated, immunoreactive, mesenchymal, and proliferative. Impressively, the gene expression signatures used to generate these subtypes were applied to a non-overlapping, publicly available repository of HGSOC gene expression and generated highly comparable clusters, validating the signatures and the four subtype classifications. While OS was not significantly different between the subtypes, further analysis generated an expression signature of 193 genes that predicted OS: 85 associated with good survival and 108 correlated with poor survival. This survival signature was similarly validated with independent datasets.
A reasonable goal is the further scrutiny, development, and refinement of TCGA data to establish a ‘PAM50’-type paradigm for ovarian cancer. The PAM50 gene signature (79) uses the level of expression of 50 genes in breast cancer biopsies or resections to classify a tumor as one of four intrinsic subtypes (luminal A, luminal B, HER2-enriched, or basal-like), a classification proven to have significant value in both treated and untreated patients for both prognosis and individualized likelihood of disease recurrence (80-82). This is a realistic and attainable goal for TCGA HGSOC data that can be used to begin to personalize treatment options based on genetic and epigenetic landscapes and perhaps increase OS.
Unlike the breast cancer PAM50 signatures, however, the four identified subtypes of HGSOC do not reveal any immediately useful or distinct therapeutic targets—at least not yet. So far, the subtyping has not revealed any novel or pervasive ‘smoking gun’ targets in HGSOC that are assailable by currently-available antineoplastics—i.e., no single, causally-dysregulated receptor tyrosine kinase, topoisomerase, or other enzymatic activity presents as an overt Achilles heel to be used for increased clinical outcomes. However, while there is no immediate therapeutic revolution in the TCGA HGSOC analysis, the identified subtypes are still well-poised to pay immediate, intermediate, and long-term dividends. In the near future, patient screening and assignment to one of these disease subtypes could prove useful in helping predict overall prognosis, which in turn may facilitate decision-making for both physician and patient. With more development and validation, the subtyping of HGSOC patients may inform chemotherapeutic choices (e.g., dosing and/or scheduling of platinum therapy; inclusion on trials using epigenetic regulators such as azacytidine and zebularine). Finally, with a considerable amount of additional, focused study of their underlying molecular and cellular biology, HGSOC subtypes may facilitate the identification of discrete molecular etiologic targets that will allow development of rationally-designed and/or disease-specific therapeutics for ovarian cancer.
Clinical implications of BRCA1 and BRCA2 status
Several recent studies have used models from previous research powered by the TCGA to reveal important connections between BRCA mutations in sporadic ovarian cancer and the impact on patient survival and treatment strategy. The breast cancer 1, early onset (BRCA1) gene locus on chromosome 17 was first linked to inherited susceptibility of early-onset breast cancer in 1990 (83). A second familial breast cancer gene (BRCA2) was later found on chromosome 13 (84). The relationship between familial risks for breast cancer with that of ovarian cancer led to studies showing that hereditary ovarian cancer is also associated with BRCA1/2 mutations (85). The strongest risk factors for ovarian cancer is a family history of the disease, and mutations in either BRCA1 or BRCA2 are found in the majority of patients with hereditary ovarian cancer (86).
The proteins encoded by BRCA1 and BRCA2 are both important in the cell response to DNA damage, but they have distinct functions. BRCA1 has diverse roles in several DNA repair pathways including homologous recombination and cell cycle checkpoint regulation where it primarily acts as a scaffold protein. BRCA2 is a DNA-binding protein that functions through its direct interaction with the homologous recombinase RAD51 (87). Both proteins are tumor suppressors, and mutations that result in functional loss of either protein increase genomic instability or a hypermutator phenotype, the so-called “BRCAness” (88). Because of their similar impact on homologous recombination, they are often referred to interchangeably, however the difference in their biology is clear and has only recently become appreciated after analysis of large datasets. While the mutation rate for either BRCA1 or BRCA2 in the general population is approximately 1 in 500, 10% of women diagnosed with EOC have germline BRCA1 or BRCA2 mutations (89). The TGCA ovarian cancer project evaluated the impact of BRCA1/2 genes and homologous recombination by evaluating mRNA expression, miRNA expression, promoter methylation, and DNA copy number in nearly 500 HGSOC. The results revealed that 20% of HGSOC have germline (17%) or somatic (3%) mutations in BRCA1/2, and an additional 11% have lost BRCA1 expression through DNA hypermethylation. Perhaps more significant with respect to clinical impact was the pathway analysis that indicated at least one genetic or epigenetic defect in genes associated with homologous recombination in half of the cancers (2).
An association between BRCA1/2 mutations and improved survival in ovarian cancer has been shown consistently in small-scale studies, reviewed by Liu et al. (90). Because of the rareness of individual non-germline BRCA1/2 mutations in ovarian cancer, these studies were unable to differentiate the individual impacts of BRCA1 and BRCA2 until data from large scale studies such as TCGA were available for analysis. The TCGA group reported that a univariate analysis of BRCA1/2 mutation status showed improved OS, however no improvement was observed in cases where BRCA1 was epigenetically silenced (2). A focused follow-up study using the TCGA data found that only mutation in BRCA2 was significantly correlated with improved survival. This study also demonstrated that cases harboring BRCA2, but not BRCA1 mutations had more genomic instability, higher primary chemotherapy sensitivity, and longer platinum-free duration (91). A separate study combining TCGA data with 20 additional studies concluded that BRCA1 mutations may also confer a survival advantage, but that this advantage is dependent on the site of mutation within BRCA1 (92). Together these data found that BRCA2 carriers exhibit a 52% 5-year OS compared with 44% for BRCA1 carriers and 36% for non-carriers. With respect to clinical implications, these data suggest that BRCA2 status may represent a phenotype of genomic instability that would predict better response to chemotherapy.
The progress in understanding BRCA gene profiles and their phenotypic outcome has an important impact on choosing a therapeutic strategy. The combination of platinum and taxane has been the standard of care for treatment of advanced ovarian cancer ever since a landmark randomized study showed improved patient survival over cisplatin-cyclophosphamide (93). Mutations in BRCA1/2 correlate with hypersensitivity to platinum agents which likely accounts for the better overall prognosis for those ovarian and breast cancer patients with germline or somatic BRCA1/2 mutations.
The recognition that 50% of high grade serous ovarian cancers have either BRCA1/2 mutations or other related defects in homologous recombination led to clinical trials with poly (ADP-ribose) polymerase (PARP) inhibitors. PARP inhibitors block base excision repair, which promotes apoptosis in cells that lack effective homologous recombination due to synthetic lethality. Early trials using the PARP inhibitor, olaparib, were successful in cases with germline BRCA1/2 mutations in ovarian, but not breast cancer (18). In December 2014, olaparib (94-96) and a companion diagnostic test, BRACAnalysis CDx (that detect the presence of mutations in the BRCA genes in blood samples from patients with ovarian cancer) were approved for women with advanced ovarian cancer associated with defective BRCA genes. The activity of olaparib was examined in 137 patients with ovarian cancer and mutated BRCA gene. Thirty-four percent of patients had a complete or partial response (PR), lasting for an average of 7.9 months. Side effects include nausea, fatigue, vomiting, diarrhea, dysgeusia, dyspepsia, headache, nasopharyngitis, cough, arthralgia, myalgia, back pain, dermatitis, pancytopenia, and abdominal pain. Potential serious side effects included myelodysplastic syndrome and acute myeloid leukemia, and lung inflammation. More recent and ongoing studies have shown additional effectiveness of PARP inhibitors in non-germline BRCA1/2-related and sporadic high-grade serous ovarian cancers (19,20). The differences in biology of BRCA1 and BRCA2, as well as the large-scale studies showing increased chemotherapeutic response in tumors with BRCA2 mutation over BRCA1 infer that BRCA2 status will be a better predictor of clinical outcome in patients treated with PARP inhibitors (21).
Dysregulation of pathways as opportunities for targeted therapies
TCGA has enlighten the role of DNA mutations in the ovarian cancer landscape. The following pathways have been found to have potentially actionable mutations in the TCGA analysis, and are proposed in decreasing order of potential importance, prioritized based on the p53 and BRCA discussions. The perspective from the literature is also included to help understand the utility of pathway targeting.
Forkhead box protein M1 (FoxM1) network
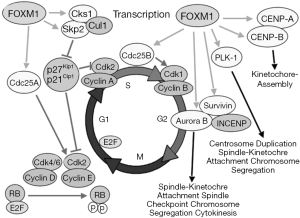
The stability of the ovarian epithelial cell genome is centrally regulated by p53, as described above. Once p53 becomes dysregulated, a cascade of events leads to multiple alterations that usually coalesce into BRCA dysfunction, also described above. Forkhead box M1 (FOXM1) is a central pivot of stability in nuclear divisions, and was found to be upregulated in p53 mutated HGSOC. FOXM1 is a transcription factor, member of the forkhead family, and it induces the expression of genes involved in the execution of mitosis. FoxM1 is regulated by p53 (97), and perhaps also regulates p53 to maintain homeostasis of mitosis (98).
Through siRNA experiments, FOXM1 expression levels positively correlate with its transcriptional targets, Cdc25B and Aurora B kinase, and negatively with p27, an indirect target of FOXM1 (via suppression of Skp2) (Figure 1) (100). The TCGA analysis found an upregulation of Aurora kinase B expression (101). Others have also described overexpression of Aurora kinase A (102). Both Aurora kinases cooperate in the mitosis process through association with microtubules during chromosome movement and segregation in a cell cycle dependent manner. Aurora kinase B localizes to specialized microtubules called K-fibers, near kinetochores, and Aurora kinase A (MIM 603072) localizes to centrosomes (103,104). Expression of Aurora A reaches a maximum at the G2-M cell cycle transition, whereas Aurora B protein is most active during mitosis. Aurora B and C (Aurora C is mainly expressed in testes) are chromosomal passenger proteins and complex with three other proteins; survivin, borealin, and INCENP. The complex is required for proper mechanism of action (105). Additionally, topoisomerase II has been implicated in the regulation of Aurora B localization and enzymatic activity (106).
Only Aurora kinase A inhibitors have been studied in ovarian cancer patients. Unfortunately, there was no selection for overexpression (107). In a single-arm phase II study of 31 patients with platinum-resistant EOC treated with alisertib (MLN8237), an Aurora A kinase inhibitor, 10% of patients achieved a PR and 20% of patients achieved stable disease (SD) lasting for longer than 3 months. Alisertib (MLN8237) was administered orally twice daily at a dose of 50 mg for 7 days in 21-day cycles. Grade 3 drug-related adverse events (AEs) included neutropenia (42%) with 6% febrile neutropenia, stomatitis (19%), and thrombocytopenia (19%). One Aurora B/C kinase inhibitor, GSK1070916A, has been tested in patients with solid tumors and the only responding patient had ovarian cancer (108). Other pan-Aurora kinase inhibitors are in clinical trials. AMG 900 is such a compound where an ovarian cancer patient had the best response (Table 2) (109). However, the observed activity remains low and aligned with common chemotherapy agents. Future trials might benefit from increased selection to match patient target with the correct inhibitor.
CDC25 phosphatase activates cyclin-dependent kinase (CDK) complexes by dephosphorylation. In humans, there are three CDC25s labeled A (110), B and C. CDC25 B together with polo-like kinase 1 (PLK1) helps in regulating the resumption of cell cycle progression after DNA damage-dependent checkpoint arrest in G2. PLK1 regulates relocation of CDC25B from the cytoplasm to the nucleus, leading to CDC25B-induced mitotic entry (111). Thus, inhibitors of PLK1 indirectly can block the overexpression of CDC25B seen in ovarian cancer. The PLK1 inhibitor, volasertib, has been studied in patients with platinum resistant ovarian cancer in a phase 2 randomized study comparing it to best standard chemotherapy. Overall responses were similar to the best chemotherapy with a PR rate of 13% and a SD rate of 44%. AEs were manageable and six patients treated with volasertib remained progression-free after 1 year on treatment compared to no patients on standard chemotherapy (112).
MDS1 and EVI1 complex locus protein EVI1 (MECOM)
The protein encoded by MECOM is a transcriptional regulator and oncoprotein that may be involved in hematopoiesis, apoptosis, development, cell differentiation, and proliferation. The encoded protein can interact with CTBP1, SMAD3, CREBBP, KAT2B, MAPK8, and MAPK9. This gene can undergo translocation with the AML1 gene, resulting in overexpression of this gene and the onset of leukemia. Several transcript variants encoding a few different isoforms have been found for this gene. Diseases associated with MECOM include 3q21q26 syndrome (a subtype of leukemia and a somatic myelodysplastic syndrome). The gene ontology (GO) annotations related to this gene include protein homodimerization activity and sequence-specific DNA binding transcription factor activity. MECOM also interacts with both classes (class I and class II) of histone deacetylases (HDAC), which abrogate the assembly of MECOM in nuclear speckles. Inhibitors of HDAC could lead to acetylation of this complex transcription factor to induce its proper function (113). Inhibitors of HDAC have not been systematically studied in ovarian cancer. Possibly reflecting effects from epigenetic modulation of this transcription factor is the study of aza and erlotinib that showed activity in patients with platinum resistant ovarian cancer (114). The HDAC inhibitor vorinostat has also been studied but lacked activity (115).
Cyclins and CDK
Progression through the cell cycle involves coordinated activation of CDK proteins that bind to their partner cyclins. Kinases (CDK4, CDK6, CDK2, and CDC2) are successively expressed, along with their partner cyclins (cyclins D, E, A, and B) as cells go through mitosis. Cyclins function as regulators of CDK kinases. Different cyclins exhibit distinct expression and degradation patterns which contribute to the temporal coordination of each mitotic event.
Cyclin E1 (CCNE1) and CDK2 have been found amplified in the TCGA analysis of ovarian cancer and other studies (116,117). CCNE1 is specifically expressed during the G1/S phase transition. CCNE1 forms a complex with and functions as a regulatory subunit of CDK2, whose activity is required for cell cycle G1/S transition. Overexpression of CCNE1 results in chromosome instability, and might contribute to tumorigenesis. CCNE1 is also involved in phosphorylation of NPAT protein (nuclear protein mapped to the ATM locus), which participates in cell-cycle regulated histone gene expression and plays a critical role in promoting cell-cycle progression in the absence of pRB (26). New inhibitors of CDK are being tested in clinical trials. Currently, only CDK4/6 has been clinically targeted, including palbociclib, LEE011 (ribociclib), abemaciclib, milciclib (118), SNS-032, TG02 (119), and seliciclib (27). Minimal information is available for ovarian cancer (Table 2) (28).
Telomerase reverse transcriptase (TERT)
Telomerase is a ribonucleoprotein polymerase that maintains telomere ends by addition of the telomere repeat TTAGGG. The enzyme consists of a protein component with reverse transcriptase activity, encoded by TERT, and an RNA component which serves as a template for the telomere repeat. Telomerase repression in adult somatic cells results in progressive shortening of telomeres, called “erosion of telomeric sequences”, which leads to cellular senescence. Cancers have been known to overexpress telomerase to avoid senescence through various mechanisms (locus variants, promoter activity) (120,121). Alternative splicing encoding different isoforms might regulate telomerase activity. Telomerase activity is regulated by a number of factors including telomerase complex-associated proteins, chaperones and polypeptide modifiers.
There are few studies of telomerase inhibitors. Imetelstat is a covalently-lipidated 13-mer thiophosphoramidate oligonucleotide that acts as a potent specific inhibitor of telomerase. It binds with high affinity to the template region of the RNA component of human telomerase (hTERC) and is a competitive inhibitor of telomerase enzymatic activity (122). Telomerase-specific CD4+ and CD8+ T cell responses can be induced upon vaccination with hTERT-transfected dendritic cells and vaccination with telomerase derived peptide, GV1001, induces T cell responses in patients with solid tumors (123). Neither of these approaches have been studied in ovarian cancer.
Nitric oxide synthase 3 (NOS3)
The free radical nitric oxide is involved in ovarian carcinogenesis by reacting to carcinogenics, modulating apoptosis and possibly by promoting growth, invasion, and metastasis (124). Of all studied polymorphisms, only one mutant allele of intron 4 (27-bp repeat in intron 4) was associated with advanced tumor stage and positive lymph node involvement. This protein is so ubiquitous that the implication of NOS3 amplification is not clear in ovarian cancer. Additionally, platinums regulate the NOS isoforms in ovarian cancer. Endogenous NOS3/NOS1 activity in platinum-resistant ovarian cancer cells produces low-level NO that protects against apoptosis. However, NOS2 contributes to platinum-induced apoptosis. These three NOS isoforms are regulated differentially by platinum agents in resistant and sensitive ovarian cancer cells. Inhibition of all NOS isoforms in platinum-resistant cells dramatically increases apoptosis (125). The inhibition of NOS can be accomplished by arginine depletion or by Nω-amino-L-arginine (LNAA) a NOS3 inhibitor. The only human studies of arginine inhibition were done with intradermal microdialysis to study vasodilation. Another approach, which was tested in hepatocellular carcinoma, involved the use of arginase. In this study, pegylated recombinant human arginase (Peg-rhAgr1) was well tolerated. A weekly dose of 1,600 U/kg induced arginine depletion and prolonged progression free survival was noted for patients achieving arginine depletion (126).
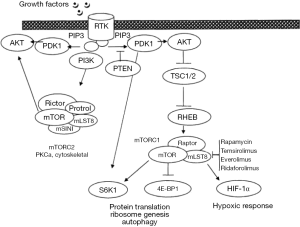
PI3K pathway
As shown in Table 1, many proteins are involved in the PI3K pathway (Figure 2). Many drugs are being tested to block one or more kinase domain of various molecules. PI3K/AKT/mTOR pathway inhibitors fall into four main categories; mTORc1 and/or mTORC2 inhibitors, PI3K inhibitors, dual mTOR/PI3K inhibitors, and AKT inhibitors. Most studies are dose or schedule finding, enrolling patients with various cancers. The evidence for activity in patients with ovarian cancer has been minimal. The pan-PI3Kinase inhibitors, especially pictilisib, have demonstrated some activity against ovarian cancer (128). Further trials should select the population with the molecular anomalies that could be targeted by this class of agents, however, the number of patients with somatic mutations within their ovarian cancer is <2%. Specific trials in a selected population would not be feasible, and these patients are better enrolled in basket trials matching patients with a rare mutation, regardless of tumor histology, to a drug expected to work through the mutated pathway.
DNA polymerase B (POLB)
The protein encoded by this gene is a DNA polymerase involved in base excision and repair. The encoded protein, acting as a monomer, is normally found in the cytoplasm, but translocates to the nucleus upon DNA damage. There are several transcript variants of this gene. Pol β is crucial for the maintenance of genomic stability (129). Pol β transcription and protein levels are increased in many cancers, including ovarian cancer. Overexpression of Pol β results in aneuploidy, thus accurate regulation of Pol β expression is essential for genome stability. POLB has also been called the “platinum resistance gene” because low expression results in increased susceptibility to platinum (130). Purified POLB incorporates the nucleotide analogues 2’-3’ deoxycytidine (ddC)-triphosphate and 3’-azido-3’-deoxythymidine (AZT)-triphosphate into DNA, causing chain termination (131). There is no known drug targeting POLB in cancer therapy.
Myostatin (MSTN)
MSTN is a secreted growth factor expressed in skeletal muscle and adipose tissue that negatively regulates skeletal muscle mass. Null MSTN mice have increase in muscle mass, reduction in fat mass, and resistance to diet-induced and genetic obesity. MSTN propeptide (MPRO) and follistatin are experimental inhibitors of MSTN which are being studied for muscle regeneration. There is very little understanding of the relation of MSTN and ovarian cancer. Interestingly, follistatin was first isolated from the ovary and is known to suppress follicle-stimulating hormone (132). One gene transfer study of follistatin is open for muscular dystrophy (NCT01519349).
Epidermal growth factor receptors (EGFR)
The human EGFR2/neu (HER2/neu) is overexpressed in about 11% of patients with high grade HGSOC (36,37). A phase 2 clinical trial of the monoclonal anti-HER2 antibody, trastuzumab, in patients overexpressing HER2 by immunohistochemistry (IHC) proved to be very difficult to implement, requiring screening of over 800 women to diagnose less than 100 with overexpression. Of these, less than half were eligible for the trial. Trastuzumab resulted in minimal therapeutic benefit with a 7% remission rate. One of the three responding patients was a long term survivor. When the fluorescence in situ hybridization (FISH) test was added to select patients by HER2 amplification, the number of eligible patients decreased to 6%; however, the sensitivity to trastuzumab increased with a 40% response rate (84). Trials of unselected patients testing pertuzumab, another monoclonal antibody or the dual EGFR/HER2 inhibitor, lapatinib, have been negative (39-41,133). However, targeting patients with specifically HER2 over-expression and/or HER3 down-regulation might be worthwhile (134). Neratinib and afatinib are newer multi-tyrosine kinase inhibitors with EGFR inhibitory activity. Today, with improved molecular techniques, the search for less common molecular endpoints might become a necessity to deliver optimal care. Inhibition of other members of this family (erb1, erb3, and erb4) is under clinical investigation, such as MM-121 (SAR256212), a fully human monoclonal antibody that targets the HER3 receptor (NCT01447706).
Signal transducer and activator of transcription (STAT) pathway
While there is some investigation of STAT3 in ovarian cancer (99), much less is known about STAT1 and STAT4 overexpression. STAT1 has been linked to platinum resistance. STAT proteins function downstream of JAKs and MAPKs, which induce the dimerization of STAT proteins, thereby allowing the translocation of STAT proteins into the nucleus (135). Jak inhibitors have only been studied in vitro in ovarian cancer models (136). STAT1 is best known for its pro-apoptotic role in response to interferons, but STAT1 has also been reported to have a pro-survival role in some cancers (137). STAT1 might be regulated by HDACs which remove an acetyl group on STAT1, which leads to cancer cell survival and resistance to platinum as was noted earlier under MECOM.
Insulin-like growth factor 1 receptor (IGF1R)
IGF1R is a member of the receptor tyrosine kinase family. Upon activation by its ligands (IGF-I or IGF-II), IGF1R phosphorylates tyrosine residues on two major substrates, insulin receptor substrate 1 (IRS-1) and SH2 domain-containing oncogenic protein (Shc), which signal through the RAS/RAF and the phosphatidylinositol 3’-kinase (PI3K)/AKT pathways (138). Without IGF1R, cells cannot transform, as shown in IGF1R knockout mice which are resistant to transformation by various viral and cellular oncogenes (139). Ganitumab (AMG 479), a human monoclonal antibody against IGF1R, has shown preclinical activity in ovarian cancer cell lines that did not have a mutated PI3K or RAS/RAF pathway (140). The drug is in clinical trials but not in ovarian cancer. The phase 1 study showed activity in the Ewing family of sarcomas.
Vascular endothelial growth factor A (VEGFA)
The VGF/VGFR pathway has been studied intensively in ovarian cancer (141). The only drug that has recently received FDA approval is bevacizumab for patients with platinum resistant ovarian cancer (42). In first and second line treatment, where bevacizumab was studied in multiple randomized studies, there was no improvement in survival despite an improvement in progression free survival (142-144). Additionally, many tyrosine kinase inhibitors of VEGFR have been studied But similarly, most studies have not shown an OS benefit: trebananib (145), sunitinib (146), nintedanib (147), sorafenib (148), and pazopanib (149). Cediranib might be effective in patients with platinum sensitive recurrent ovarian cancer, but the full report has not been published (150).
The most benefit of anti-angiogenic drugs seems to be for patients with platinum resistant ovarian cancer, and the addition of an antiangiogenic agent comes at the expense of increased toxicity (43).
Heat shock protein 90kda alpha (cytosolic), class B member 1 (HSP90AB1)
The molecular chaperones, Hsp90 and Hsp70, are involved in the folding and maturation of key regulatory proteins, such as transcription factors, kinases, and others that are involved in cancer progression (151). Client oncoproteins include EGFR, Her2/neu, Akt, c-RAF, IGFR, and others. The chaperones interact with these regulatory proteins to help conformation, transportation and degradation through ubiquitination. Chaperones (named by their molecular weight) ensure the maintenance of a functional proteome under normal and stress conditions (152).
The only targetable chaperone thus far has been Hsp90. The Hsp90 inhibitor, geldanamycin, proved to be ineffective in cancer. Tanespimycin has been tested in ovarian cancer in patients receiving concurrent gemcitabine with limited activity (46). While changes are observed among client proteins with Hsp90 inhibition, this inhibition has also resulted in a prolonged increase in Hsp70, which can lead to resistance to Hsp90 inhibition (153). Inhibitors of Hsp70 have been notoriously difficult to design (127).
Epithelial cellular adhesion molecule (EpCAM)
EpCAM is a transmembrane glycoprotein mediating Ca2+-independent homotypic cell-cell adhesion exclusively in epithelial cells. EpCAM may upregulate c-myc and cyclins A & E and play a role in tumorigenesis and metastasis. Immunotherapeutic trials have been completed in patients with ovarian cancer. Catumaxomab, a monoclonal antibody against CD3 and EpCAM, has been tested for the treatment of refractory ascites (50) and improves quality of life. It is approved in Europe for refractory ascites, but not in the US.
TITIN (TNN)
When the TGCA analysis searched for driver mutations, 518 genes were ranked from highest probability to lowest. For example, BRAF and serine/threonine kinase 11 (STK11) were ranked second and sixteenth. TTN ranked number one with 63 non-synonymous and 13 synonymous mutations. Half of the non-synonymous mutations in TTN are likely to be passenger mutations. TTN has been involved with muscle contractility. It is the largest polypeptide encoded by the human genome. TTN is expressed in many cell types and interferes with chromosomal structure and elasticity that could be compatible with a role in oncogenesis (154). The role of TTN as a cancer gene is currently a mathematically based prediction, and will require direct biological evaluation (155).
RAS/RAF/MEK pathway
This pathway has been extensively studied in cancer and is currently being therapeutically targeted with great success. Mutations in proteins that activate this pathway are labeled driver mutations. The most famous one in melanoma is the BRAF V600E. BRAF and RAS mutations seem mutually exclusive. The BRAF inhibitors, vemurafenib and dabrafenib, and the MEK inhibitor, trametinib are approved for the treatment of melanoma. Many other tyrosine kinases inhibitors are under clinical trials. This pathway has not been found to be activated in most high grade HGSOC, contrarily to the low grade HGSOC where RAS or BRAF mutations are detected (156). Patients with NF1 mutations might have an activation of the MAPK pathway (118). Again, the recommendation is to enroll the rare patients with one such mutation in basket trials, matching mutated proteins to specific tyrosine kinases.
Conclusions
The in-depth analysis done by the TCGA Atlas project on HGSOC has revealed known and previously unknown targets that are usually normal cellular processes gone ‘berserk’ by changes in the ballet of protein interactions. These changes are due to various mechanisms, ranging from DNA mutations, mitotic machinery, dysregulation of signaling pathways, and epigenetic modifications that prevent homeostasis in the affected cells. These dysregulations involve mainly DNA repair (in about 50% of ovarian cancers) or transcription alterations of critical proteins. Interestingly, no specific protein disruption that is known to affect the immune recognition of ovarian cancer by T cells and other killer immune cells has been identified. Despite some effectiveness of antiangiogenic therapy, very few HGSOC cases are intrinsically by alterations of the basic angiogenic protein machinery. The data from the TCGA has been integrated with extensive ovarian cancer molecular studies in the hopes of leading to improved understanding of ovarian cancer biology. Much remains to be investigated and further research must probe the interactome that pushes normal cells to descend into the trap of immortality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Franco Muggia and Eleonora Teplinsky) for the series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.02.01). The series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolv
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071-8. [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. [PubMed]
- Buller RE, Runnebaum IB, Karlan BY. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther 2002;9:553-66. [PubMed]
- Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett 2014;588:2622-7. [PubMed]
- Rahma OE, Ashtar E, Czystowska M, et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother 2012;61:373-84. [PubMed]
- Vermeij R, Leffers N, Hoogeboom BN, et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: a single-arm phase II study. Int J Cancer 2012;131:E670-80. [PubMed]
- Wolf JK, Bodurka DC, Gano JB, et al. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum- and paclitaxel-resistant epithelial ovarian cancer. Gynecol Oncol 2004;94:442-8. [PubMed]
- Zeimet AG, Marth C. Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol 2003;4:415-22. [PubMed]
- Mencalha AL, Binato R, Ferreira GM, et al. Forkhead box M1 (FoxM1) gene is a new STAT3 transcriptional factor target and is essential for proliferation, survival and DNA repair of K562 cell line. PLoS One 2012;7:e48160 [PubMed]
- Hetland TE, Nymoen DA, Holth A, et al. Aurora B expression in metastatic effusions from advanced-stage ovarian serous carcinoma is predictive of intrinsic chemotherapy resistance. Hum Pathol 2013;44:777-85. [PubMed]
- Ma YX, Li XZ. Effect of aurora kinase B inhibitor AZD1152 in the treatment of cisplatin-resistant ovarian carcinoma. Zhonghua Fu Chan Ke Za Zhi 2013;48:46-50. [PubMed]
- Vivas-Mejia PE, Rodriguez-Aguayo C, Han HD, et al. Silencing survivin splice variant 2B leads to antitumor activity in taxane--resistant ovarian cancer. Clin Cancer Res 2011;17:3716-26. [PubMed]
- Shapiro GI, Tibes R, Gordon MS, et al. Phase I studies of CBP501, a G2 checkpoint abrogator, as monotherapy and in combination with cisplatin in patients with advanced solid tumors. Clin Cancer Res 2011;17:3431-42. [PubMed]
- Jimeno A, Li J, Messersmith WA, et al. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol 2008;26:5504-10. [PubMed]
- Schöffski P, Blay JY, De Greve J, et al. Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network Of Core Institutes (NOCI). Eur J Cancer 2010;46:2206-15. [PubMed]
- Valsasina B, Beria I, Alli C, et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther 2012;11:1006-16. [PubMed]
- Weichert W, Denkert C, Schmidt M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer 2004;90:815-21. [PubMed]
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-34. [PubMed]
- Clamp A, Jayson G. PARP inhibitors in BRCA mutation-associated ovarian cancer. Lancet Oncol 2015;16:10-2. [PubMed]
- Burgess MA, Puhalla SL. BRCA 1/2-mutation related and sporadic breast and ovarian cancers: More alike than different. Frontiers in Oncology 2014;4:19. [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-92. [PubMed]
- Chakraborty S, Senyuk V, Sitailo S, et al. Interaction of EVI1 with cAMP-responsive element-binding protein-binding protein (CBP) and p300/CBP-associated factor (P/CAF) results in reversible acetylation of EVI1 and in co-localization in nuclear speckles. J Biol Chem 2001;276:44936-43. [PubMed]
- Abe MK, Saelzler MP, Espinosa R 3rd, et al. ERK8, a new member of the mitogen-activated protein kinase family. J Biol Chem 2002;277:16733-43. [PubMed]
- Colecchia D, Strambi A, Sanzone S, et al. MAPK15/ERK8 stimulates autophagy by interacting with LC3 and GABARAP proteins. Autophagy 2012;8:1724-40. [PubMed]
- Rossi M, Colecchia D, Iavarone C, et al. Extracellular signal-regulated kinase 8 (ERK8) controls estrogen-related receptor alpha (ERRalpha) cellular localization and inhibits its transcriptional activity. J Biol Chem 2011;286:8507-22. [PubMed]
- Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res 2011;17:1591-602. [PubMed]
- Benson C, White J, De Bono J, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer 2007;96:29-37. [PubMed]
- Shapiro G, et al. A first-in-human phase I study of the CDK 4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol 2013;31:A2500.
- Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2012;18:568-76. [PubMed]
- Rocca A, Farolfi A, Bravaccini S, et al. Palbociclib (PD 0332991): targeting the cell cycle machinery in breast cancer. Expert Opin Pharmacother 2014;15:407-20. [PubMed]
- Kellogg DL Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol (1985) 2009;107:1438-44. [PubMed]
- Westin SN, Herzog TJ, Coleman RL. Investigational agents in development for the treatment of ovarian cancer. Invest New Drugs 2013;31:213-29. [PubMed]
- Available online: http://www.proteinatlas.org/ENSG00000138379-MSTN/cancer/tissue/ovarian+cancer
- Ma J, Lyu H, Huang J, et al. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 2014;13:105. [PubMed]
- Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer 2011;104:1241-5. [PubMed]
- Bookman MA, Darcy KM, Clarke-Pearson D, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol 2003;21:283-90. [PubMed]
- Teplinsky E, Muggia F. Targeting HER2 in ovarian and uterine cancers: challenges and future directions. Gynecol Oncol 2014;135:364-70. [PubMed]
- Ray-Coquard I, Guastalla JP, Allouache D, et al. HER2 Overexpression/Amplification and Trastuzumab Treatment in Advanced Ovarian Cancer: A GINECO Phase II Study. Clinical Ovarian Cancer 2008;1:54-9.
- Garcia AA, Sill MW, Lankes HA, et al. A phase II evaluation of lapatinib in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol 2012;124:569-74. [PubMed]
- Lheureux S, Krieger S, Weber B, et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. Int J Gynecol Cancer 2012;22:1483-8. [PubMed]
- Rivkin SE, Mullerc C, Malmgren JA. A Phase I/II Study of Lapatinib Plus Carboplatin and Paclitaxel in Relapsed Ovarian and Breast Cancer. Clinical Ovarian Cancer 2009;2:112-7.
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32:1302-8. [PubMed]
- Verschraegen CF, Czok S, Muller CY, et al. Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann Oncol 2012;23:3104-10. [PubMed]
- Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 2011;71:1397-412. [PubMed]
- Stockler MR, Hilpert F, Friedlander M, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol 2014;32:1309-16. [PubMed]
- Hendrickson AE, Oberg AL, Glaser G, et al. A phase II study of gemcitabine in combination with tanespimycin in advanced epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol 2012;124:210-5. [PubMed]
- Banerji U, Sain N, Sharp SY, et al. An in vitro and in vivo study of the combination of the heat shock protein inhibitor 17-allylamino-17-demethoxygeldanamycin and carboplatin in human ovarian cancer models. Cancer Chemother Pharmacol 2008;62:769-78. [PubMed]
- Hubbard J, Erlichman C, Toft DO, et al. Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Invest New Drugs 2011;29:473-80. [PubMed]
- Jhaveri K, Miller K, Rosen L, et al. A phase I dose-escalation trial of trastuzumab and alvespimycin hydrochloride (KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin Cancer Res 2012;18:5090-8. [PubMed]
- Wimberger P, Gilet H, Gonschior AK, et al. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol 2012;23:1979-85. [PubMed]
- Burges A, Wimberger P, Kümper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res 2007;13:3899-905. [PubMed]
- Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 2010;36:458-67. [PubMed]
- Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 2008;14:1351-6. [PubMed]
- Bösmüller H, Fischer A, Pham DL, et al. Detection of the BRAF V600E mutation in serous ovarian tumors: a comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum Pathol 2013;44:329-35. [PubMed]
- Despierre E, Yesilyurt BT, Lambrechts S, et al. Epithelial ovarian cancer: rationale for changing the one-fits-all standard treatment regimen to subtype-specific treatment. Int J Gynecol Cancer 2014;24:468-77. [PubMed]
- Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012;30:282-90. [PubMed]
- Matulonis U, Wulf GM, Birrer MJ, et al. Phase I study of oral BKM120 and oral olaparib for high-grade serous ovarian cancer (HGSC) or triple-negative breast cancer (TNBC). J Clin Oncol 32:5s, 2014 (suppl; abstr 2510).
- Sangha N, Wu R, Kuick R, et al. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia 2008;10:1362-72, following 1372.
- Greenblatt MS, Bennett WP, Hollstein M, et al. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994;54:4855-78. [PubMed]
- Brown CJ, Lain S, Verma CS, et al. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer 2009;9:862-73. [PubMed]
- Eischen CM, Lozano G. Mdm network and its regulation of p53 activities: a rheostat of cancer risk. Hum Mutat 2014;35:728-37. [PubMed]
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005;6:44-55. [PubMed]
- Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat 2014;35:672-88. [PubMed]
- Kamihara J, Rana HQ, Garber JE. Germline TP53 mutations and the changing landscape of Li-Fraumeni syndrome. Hum Mutat 2014;35:654-62. [PubMed]
- Bisio A, Ciribilli Y, Fronza G, et al. TP53 mutants in the tower of babel of cancer progression. Hum Mutat 2014;35:689-701. [PubMed]
- Langhe R, Norris L, Saadeh FA, et al. A novel serum microRNA panel to discriminate benign from malignant ovarian disease. Cancer Lett 2015;356:628-36. [PubMed]
- Liu L, Zou J, Wang Q, et al. Novel microRNAs expression of patients with chemotherapy drug-resistant and chemotherapy-sensitive epithelial ovarian cancer. Tumour Biol 2014;35:7713-7. [PubMed]
- Xiang Y, Ma N, Wang D, et al. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene 2014;33:378-86. [PubMed]
- Ding Q. microRNA Cancer Association Database 2014. Available online: http://mircancer.ecu.edu
- Wang D, Gu J, Wang T, et al. OncomiRDB: a database for the experimentally verified oncogenic and tumor-suppressive microRNAs. Bioinformatics 2014;30:2237-8. [PubMed]
- Roossink F, de Jong S, Wisman GB, et al. DNA hypermethylation biomarkers to predict response to cisplatin treatment, radiotherapy or chemoradiation: the present state of art. Cell Oncol (Dordr) 2012;35:231-41. [PubMed]
- Fu S, Hu W, Iyer R, et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer 2011;117:1661-9. [PubMed]
- Li Y, Hu W, Shen DY, et al. Azacitidine enhances sensitivity of platinum-resistant ovarian cancer cells to carboplatin through induction of apoptosis. Am J Obstet Gynecol 2009;200:177.e1-9.
- Glasspool RM, Brown R, Gore ME, et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br J Cancer 2014;110:1923-9. [PubMed]
- Matei D, Fang F, Shen C, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res 2012;72:2197-205. [PubMed]
- Zeller C, Dai W, Steele NL, et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene 2012;31:4567-76. [PubMed]
- Thottassery JV, Sambandam V, Allan PW, et al. Novel DNA methyltransferase-1 (DNMT1) depleting anticancer nucleosides, 4'-thio-2'-deoxycytidine and 5-aza-4'-thio-2'-deoxycytidine. Cancer Chemother Pharmacol 2014;74:291-302. [PubMed]
- Odunsi K, Matsuzaki J, James SR, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res 2014;2:37-49. [PubMed]
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160-7. [PubMed]
- Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222-32. [PubMed]
- Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 2012;18:4465-72. [PubMed]
- Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298-305. [PubMed]
- Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990;250:1684-9. [PubMed]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-92. [PubMed]
- Steichen-Gersdorf E, Gallion HH, Ford D, et al. Familial Site-specific Ovarian Cancer Is Linked to BRCA1 on 17q12-21. Am J Hum Genet 1994;55:870-5. [PubMed]
- Muggia F, Safra T. ‘BRCAness’ and Its Implications for Platinum Action in Gynecologic Cancer. Anticancer Res 2014;34:551-6. [PubMed]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68-78. [PubMed]
- Rigakos G, Razis E. BRCAness: finding the Achilles heel in ovarian cancer. Oncologist 2012;17:956-62. [PubMed]
- Whittemore AS, Gong G, Itnyre J. Prevalence and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three U.S. population-based case-control studies of ovarian cancer. Am J Hum Genet 1997;60:496-504. [PubMed]
- Liu G, Yang D, Sun Y, et al. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics 2012;13:1523-35. [PubMed]
- Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011;306:1557-65. [PubMed]
- Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012;307:382-90. [PubMed]
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1-6. [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [PubMed]
- Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 2012;30:372-9. [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J Clin Oncol 2015;33:244-50. [PubMed]
- Zhang X, Cheng L, Minn K, et al. Targeting of mutant p53-induced FoxM1 with thiostrepton induces cytotoxicity and enhances carboplatin sensitivity in cancer cells. Oncotarget 2014;5:11365-80. [PubMed]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 2007;8:440-50. [PubMed]
- Madoux F, Koenig M, Sessions H, et al. Modulators of STAT Transcription Factors for the Targeted Therapy of Cancer (STAT3 Activators). Probe Reports from the NIH Molecular Libraries Program [Internet]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK51964/
- Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol 2013;85:644-52. [PubMed]
- Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 1998;17:3052-65. [PubMed]
- Hu W, Kavanagh JJ, Deaver M, et al. Frequent overexpression of STK15/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res 2005;15:49-57. [PubMed]
- Lampson MA, Renduchitala K, Khodjakov A, et al. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol 2004;6:232-7. [PubMed]
- Wu Q, Zhang W, Mu T, et al. Aurora B kinase is required for cytokinesis through effecting spindle structure. Cell Biol Int 2013;37:436-42. [PubMed]
- Kunitoku N, Sasayama T, Marumoto T, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell 2003;5:853-64. [PubMed]
- Coelho PA, Queiroz-Machado J, Carmo AM, et al. Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol 2008;6:e207 [PubMed]
- Matulonis UA, Sharma S, Ghamande S, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol 2012;127:63-9. [PubMed]
- McNeish I, Anthoney A, Loadman P, et al. A phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of the selective aurora kinase inhibitor GSK1070916A. J Clin Oncol 31, 2013 (suppl; abstr 2525).
- Carducci M, et al. First-in-human study of AMG 900, an oral pan-Aurora kinase inhibitor, in adult patients (pts) with advanced solid tumors. J Clin Oncol 2012;30:A3009.
- Hu W, Wu W, Nash MA, et al. Anomalies of the TGF-beta postreceptor signaling pathway in ovarian cancer cell lines. Anticancer Res 2000;20:729-33. [PubMed]
- Lobjois V, Jullien D, Bouché JP, et al. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim Biophys Acta 2009;1793:462-8.
- Pujade-Lauraine E, Weber BE, Ray-Coquard I, et al. Phase II trial of volasertib (BI 6727) versus chemotherapy (CT) in platinum-resistant/refractory ovarian cancer (OC). J Clin Oncol 31, 2013 (suppl; abstr 5504).
- Dokmanovic M, Perez G, Xu W, et al. Histone deacetylase inhibitors selectively suppress expression of HDAC7. Mol Cancer Ther 2007;6:2525-34. [PubMed]
- Bauman J, Verschraegen C, Belinsky S, et al. A phase I study of 5-azacytidine and erlotinib in advanced solid tumor malignancies. Cancer Chemother Pharmacol 2012;69:547-54. [PubMed]
- Takai N, Narahara H. Histone deacetylase inhibitor therapy in epithelial ovarian cancer. J Oncol 2010;2010:458431.
- Bedrosian I, Lu KH, Verschraegen C, et al. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene 2004;23:2648-57. [PubMed]
- Marone M, Scambia G, Giannitelli C, et al. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer 1998;75:34-9. [PubMed]
- Weiss GJ, Hidalgo M, Borad MJ, et al. Phase I study of the safety, tolerability and pharmacokinetics of PHA-848125AC, a dual tropomyosin receptor kinase A and cyclin-dependent kinase inhibitor, in patients with advanced solid malignancies. Invest New Drugs 2012;30:2334-43. [PubMed]
- Burrows F, Goh K, Novotny-Diermayr V, et al. TG02: A novel, multi-kinase inhibitor with potent activity against solid tumors. J Clin Oncol 28, 2010 (suppl; abstr e13549).
- Bojesen SE, Pooley KA, Johnatty SE, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet 2013;45:371-84, 384e1-2.
- Beesley J, Pickett HA, Johnatty SE, et al. Functional polymorphisms in the TERT promoter are associated with risk of serous epithelial ovarian and breast cancers. PLoS One 2011;6:e24987 [PubMed]
- Thompson PA, Drissi R, Muscal JA, et al. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: a Children's Oncology Group Phase I Consortium Study (ADVL1112). Clin Cancer Res 2013;19:6578-84. [PubMed]
- Kyte JA. Cancer vaccination with telomerase peptide GV1001. Expert Opin Investig Drugs 2009;18:687-94. [PubMed]
- Hefler LA, Ludwig E, Lampe D, et al. Polymorphisms of the endothelial nitric oxide synthase gene in ovarian cancer. Gynecol Oncol 2002;86:134-7. [PubMed]
- Leung EL, Fraser M, Fiscus RR, et al. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance. Br J Cancer 2008;98:1803-9. [PubMed]
- Yau TC, Cheng PN, Chan P, et al. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics, and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. J Clin Oncol 32, 2014 (suppl; abstr e15137).
- Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 2012;325:117-24. [PubMed]
- Hoff DD, LoRusso P, Demetri GD, et al. A phase I dose-escalation study to evaluate GDC-0941, a pan-PI3K inhibitor, administered QD or BID in patients with advanced or metastatic solid tumors. J Clin Oncol 29: 2011 (suppl; abstr 3052).
- Ray S, Menezes MR, Senejani A, et al. Cellular roles of DNA polymerase beta. Yale J Biol Med 2013;86:463-9. [PubMed]
- Iwatsuki M, Mimori K, Yokobori T, et al. A platinum agent resistance gene, POLB, is a prognostic indicator in colorectal cancer. J Surg Oncol 2009;100:261-6. [PubMed]
- Bouayadi K, Hoffmann JS, Fons P, et al. Overexpression of DNA polymerase beta sensitizes mammalian cells to 2',3'-deoxycytidine and 3'-azido-3'-deoxythymidine. Cancer Res 1997;57:110-6. [PubMed]
- Phillips DJ, de Kretser DM. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol 1998;19:287-322. [PubMed]
- Kaye SB, Poole CJ, Dańska-Bidzińska A, et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann Oncol 2013;24:145-52. [PubMed]
- Langdon SP, Faratian D, Nagumo Y, et al. Pertuzumab for the treatment of ovarian cancer. Expert Opin Biol Ther 2010;10:1113-20. [PubMed]
- Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci 2000;25:496-502. [PubMed]
- Abubaker K, Luwor RB, Zhu H, et al. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer 2014;14:317. [PubMed]
- Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal 2007;19:454-65. [PubMed]
- Sepp-Lorenzino L. Structure and function of the insulin-like growth factor I receptor. Breast Cancer Res Treat 1998;47:235-53. [PubMed]
- Sell C, Dumenil G, Deveaud C, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol 1994;14:3604-12. [PubMed]
- Beltran PJ, Calzone FJ, Mitchell P, et al. Ganitumab (AMG 479) inhibits IGF-II-dependent ovarian cancer growth and potentiates platinum-based chemotherapy. Clin Cancer Res 2014;20:2947-58. [PubMed]
- Gavalas NG, Liontos M, Trachana SP, et al. Angiogenesis-related pathways in the pathogenesis of ovarian cancer. Int J Mol Sci 2013;14:15885-909. [PubMed]
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039-45. [PubMed]
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473-83. [PubMed]
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484-96. [PubMed]
- Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:799-808. [PubMed]
- Baumann KH, du Bois A, Meier W, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann Oncol 2012;23:2265-71. [PubMed]
- Ledermann JA, Hackshaw A, Kaye S, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J Clin Oncol 2011;29:3798-804. [PubMed]
- Matei D, Sill MW, Lankes HA, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol 2011;29:69-75. [PubMed]
- du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014;32:3374-82. [PubMed]
- Ledermann JA, Perren T, Raja FA, et al. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. NCRI Cancer Conference, 2013. Liverpool, UK, LB80.
- Wegele H, Müller L, Buchner J. Hsp70 and Hsp90--a relay team for protein folding. Rev Physiol Biochem Pharmacol 2004;151:1-44. [PubMed]
- Vabulas RM, Raychaudhuri S, Hayer-Hartl M, et al. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2010;2:a004390 [PubMed]
- Nowakowski GS, McCollum AK, Ames MM, et al. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin Cancer Res 2006;12:6087-93. [PubMed]
- Machado C, Andrew DJ. D-Titin: a giant protein with dual roles in chromosomes and muscles. J Cell Biol 2000;151:639-52. [PubMed]
- Bizama C, Benavente F, Salvatierra E, et al. The low-abundance transcriptome reveals novel biomarkers, specific intracellular pathways and targetable genes associated with advanced gastric cancer. Int J Cancer 2014;134:755-64. [PubMed]
- Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol 2011;42:918-31. [PubMed]


