
Robotic surgical system completed radical gastrectomy for gastric cancer after liver transplantation: case report and systematic review
Introduction
Da Vinci robot surgery system has been used in the surgery of gastric cancer (GC). The operations were performed on GC patients at early and advanced stages. The surgical methods have been developed from distal to proximal radical gastrectomy, and even in radical total gastrectomy and gastric remnant after partial gastrectomy (1,2). New cases of GC after liver transplantation are rarely reported, and no robotic surgery has been reported so far. Only a few cases have been reported, and radical resection combined with comprehensive treatment remains the major therapeutic method. One case of laparoscopic-assisted radical resection has been reported in Korea. In this study, with the assistance of robotic GC surgery (3), radical GC surgery was successfully performed on two patients after liver transplantation at Southwest Hospital on October 23 and December 25, 2018. Follow-up examination was carried out 6 and 4 months after surgery, respectively, and the therapeutic outcome was good. Written informed consent was obtained from each patient prior to operation, and the information of patients was anonymous. We present the following case in accordance with the CARE reporting (available at http://dx.doi.org/10.21037/tcr-19-1786).
Case presentation
Case 1, male, 63 years old, was diagnosed with primary liver cancer and received orthotopic liver transplantation at Southwest Hospital in 2007. During the 11 years after surgery, the quality of his life is improved. No medical or family history of liver cancer and GC were presented. However, the patient was hospitalized and subjected to enhanced CT scan in September 2018 due to pain or discomfort centered in the upper abdomen. The results revealed irregular shape of his gastric antrum wall, and the mucosa was rigid and rough, small curvature of stomach and pylorus were scattered with enlarged lymphnode-shadows, up to ~4.0 × 2.3 cm2 in size. No recurrence and distant metastasis of hepatocellular carcinoma was detected. Gastroscopy revealed giant irregular ulcer in antrum. Additionally, the pathological diagnosis was adenocarcinoma of antrum. Physical examination revealed that the body mass index was 25.08 kg/m2, no abdominal mass or enlargement of Virchow lymph nodes was found, and there was an inverted L-shaped incision (~50 cm) in the right upper abdomen. Diagnostic results: (I) adenocarcinoma of gastric antrum; (II) postoperative complication of liver transplantation; (III) varicose veins in the upper digestive tract.
Case 2, a 64-year-old male, underwent orthotopic liver transplantation at Southwest Hospital in January 2018 due to acute-on-chronic liver failure. The quality of his life was improved in the following 11 months. No medical or family history of liver cancer and GC were presented. The patient was hospitalized and subjected to CT scan in September 2018 due to upper abdominal discomfort. The results indicated that the gastric wall in lower gastric body and antrum were unevenly thickened, and soft tissue density was increased. Mucosal disruption was also detected in the lesion area, where large ulcer (~5.0 cm in diameter) could be observed. The gastric wall of lesion area was stiff, and lesion boundary was unclear. In addition, the serosal surface was not smooth, and the lesion extended to pylorus, consequently resulting in pyloric stenosis (Figure 1A). Gastroscopy revealed giant irregular ulcer in antrum (Figure 1B). The pathological diagnosis was poorly differentiated adenocarcinoma in antrum. Physical examination revealed that the body mass index was 20.37 kg/m2, no enlargement was detected in supraclavicular lymph node, and there was an inverted L-shaped incision (~50 cm) in the right upper abdomen. Diagnostic results: (I) adenocarcinoma of gastric antrum; (II) postoperative complication of liver transplantation; (III) type II diabetes mellitus.
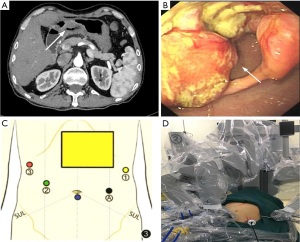
Radical subtotal gastrectomy with robotic Da Vinci system was planned for both patients.
Surgical procedure
Tracheal intubation and intravenous anesthesia were performed. The supine position was used, and the legs were separated. The abdominal wall was punctured using 5-hole method: 12 mm Trocar was inserted as the lens hole 2 cm from the lower umbilical margin, and 8 mm Trocar was placed beneath the left axillary front rib and used as the main manipulation hole of robot-arm no.1. Additionally, 12 mm Trocar was inserted 1 cm under the left clavicle midline as the assistant manipulation hole, and 8 mm Trocar was placed 3 cm beneath the right clavicle midline was with as the manipulation hole of robot-arm no.2, Furthermore, 8 mm Trocar was inserted under the right axillary front rib as the manipulation hole of robot-arm no.3. The interval between Trocars was ≥8 cm (Figure 1C,D). No ascites was found, and no metastasis was detected in peritoneum, omentum, pancreas, spleen, colon and pelvic. Both patients received orthotopic liver transplantation, and the omentum moved upward to encapsulate the porta hepatis and adhered to the abdominal wall (Figure 2A). In case 2, the left gastroepiploic membrane partially adhered to the splenic flexure and the left upper abdominal wall, and the anterior gastric wall also adhered to the abdominal wall. For the installation of Da Vinci robot system, the assembly of robot-arm no.1, no.2 and auxiliary holes was completed. The bipolar electrocoagulation of robot-arm no.2 was alternately coordinated with the hook of no.1. When the adhesion between omentum and the left upper abdominal wall was separated, the robot-arm no.3 was assembled, and the pyloric region of the antrum was exposed. The tumors were located prepyloric region of the stomach in both patients. The sizes of tumors were ~3×3 and ~4×5 cm2 in case 1 and 2 respectively, which had penetrated the serosal surface. Furthermore, the adhesion in hepatic hilum, gastric anterior wall and spleen was isolated. In addition, ascending colon, duodenum and hepatic hilum were adhered, which was completely separated using the ultrasound knife attached to robot-arm no.1 (Figure 2B,C).
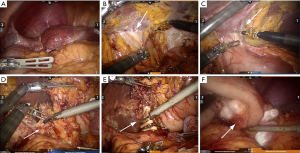
The greater omentum was lifted, and the transverse colon was separated using ultrasonic scalpel. The left gastroepiploic artery and vein were isolated, and the lymph nodes of group 4sb were dissected. Subsequently, the right gastroepiploic vessels were isolated, and the lymph nodes of group 4d, 14v and 6 were removed. Furthermore, the duodenal bulb was exposed and the duodenum was dissected using an intraluminal closure device, and stomach was rotated to the left. The common bile duct, portal vein and proper hepatic artery were encapsulated by fibrous tissues. As a result of liver transplantation, right gastric artery or vein was not observed. Lymphatic adipose tissue was dissected in group 5 and 12a. Lymph nodes of group 8a were removed along the surface of common hepatic artery at the upper edge of the pancreas (Figure 2D).
In case 1, the left gastric artery was not found, but compensatory hypertrophic arteries and blood supply in the lesser curvature from the accompanying veins were observed. After bioclips were placed, ultrasound scalpel was applied (Figure 2E). The lesser curvature was isolated along the lesser curvature side of the stomach. The lymph nodes in group 3 were notably enlarged, (~3 cm in diameter), which were subsequently removed (Figure 2F). In case 2, the left gastric artery was exposed, lymph nodes and adipose tissue of group 7, 9 and proximal splenic artery (group 11p) were dissected, and left gastric artery and vein were clamped with two Tyco bioclips and cut with ultrasound scalpel.
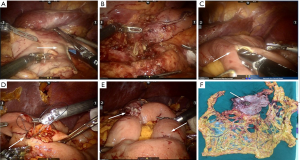
The distal gastrectomy was performed using a 60 mm cutting closure device at 1 cm beneath the lesser curvature side of antrum and above the 1/3 boundary of the greater curvature side (Figure 3A). The distal gastrectomy and isolation of D2 lymph nodes were completed (Figure 3B). The reconstruction of digestive tract was completed using type II + Braun anastomosis with the assistance of Da Vinci Si robotic surgical system. Disposable laparoscopic stapler was used to perform anterior gastrojejunal side-to-side and jejunojejunal anastomosis. The continuous closure was performed using 3-0 barbed suture (Figure 3C,D,E). Indwelling drainage was completed, and an incision (~3 cm) was made at umbilical Trocar to remove the specimen (Figure 3F).
Results
Distal subtotal gastrectomy + D2 radical lymph node dissection were successfully performed on both patients.
Case 1
The operation last for 315 minutes, and the bleeding volume was 145 mL. The urinary catheter and nasogastric tube were removed 46 and 70 hours after surgery respectively, the anal exhaust was observed 48 hours postoperatively. Semi-liquid diet was taken by the patient orally 3 days after operation, since then, immunosuppressant mofetil (1,000 mg, BID) and tacrolimus (10 mg, BID) were given. The abdominal drainage tube was removed 6 days after surgery, and the patient was discharged on day 7. No complications such as abdominal hemorrhage, wound infection, anastomotic leakage and stenosis were observed. The pathological diagnosis was medium differentiated adenocarcinoma of antrum. A total of 19 lymph nodes were analyzed and confirmed by pathologist, and metastasis was found in 11 lymph nodes. The postoperative diagnosis was moderately differentiated gastric antrum adenocarcinoma pT4aN3aM0, stage III B. The first chemotherapy was performed 3 weeks after operation using SOX, and 8 cycles of treatment were planned. Intravenous chemotherapy was carried out for three times. At present, follow-up is still in progress. During the follow-up period, the patient is generally in good health and no complications occurred.
Case 2
The operation time was 275 minutes and the bleeding volume was 125 mL. The urethra and gastric tube were removed 36 and 70 hours after surgery respectively. The anal exhaust was observed 48 hours postoperatively. Semi-liquid diet was taken by the patient orally 3 days after operation, and the treatment using mofetil (1,000 mg, BID) and tacrolimus (10 mg, BID) were resumed. The abdominal drainage tube was removed 8 days after surgery, and the patient was discharged on day 9. No postoperative complications occurred. The pathological diagnosis was poorly differentiated adenocarcinoma; 21 lymph nodes were checked by pathologist, and metastasis was detected in 2 of them. The postoperative diagnosis was pT4aN2M0, stage III A of moderately differentiated adenocarcinoma of the gastric antrum. Gastric retention was conducted since 3 weeks post-operation. Gastroscopy revealed edema in anastomotic stoma. Conservative treatment was performed using jejunal tube. After 2 weeks, normal diet was gradually restored. Chemotherapy was initiated 2 months after operation using SOX. During the follow-up period, the patient is generally in good health, but suffers from acid reflux and belching occasionally.
Anesthesia and posture were the same as robotic surgery for primary GC. In both cases, an inverted L-shaped incision (~50 cm) in the right upper abdomen was used for liver transplantation. Due to local adhesion in the right upper abdomen, pneumoperitoneum was established and the first Trocar was placed 2 cm from the bottom edge of umbilical fossa to avoid damage to the intestinal tract. The remaining 4 Trocars were punctured under visual guidance. Firstly, the puncture of the left upper abdomen was completed, and an arm was fitted with an electric hook. Subsequently, Trocar of the second and third arms on the right side of the Da Vinci rob were assembled. End-gripping forceps were used for robot-arm no.3, which allowed larger contact area with intestinal canal and dispersed the pressing force evenly without damaging intestinal wall. They can also provoke liver exposure and help to reveal visual field, thus the efficacy was improved. The 2nd arm coordinated with the 1st arm for the fine-tuned movement during the operation, which provided more flexibility. Both arms were equipped with sharp and compact grippers. The tension between the 2nd arm and the tissues isolated by the 3rd arm was appropriate, which makes it possible for the electric hook and ultrasonic scalpel on the 1st arm to operate precisely.
The estimated blood loss in two cases was 145 and 125 mL, and the patients were discharged on day 7 and 9, respectively. Both patients benefit from the minimally invasive surgery. The second patient developed mild anastomotic edema 3 weeks following operation, but was recovered 2 weeks after conservative treatment.
Discussion
The most common types of cancer occurred after liver transplantation are non-melanoma skin cancer, respiratory and digestive system tumors, as well as post-transplant lymphoproliferative disorders (4). Immunosuppressive agents could be a risk factor due to the long-term suppression of immune system, which makes it easier for the mutated tumors to escape the immune surveillance, consequently leading to the formation of malignant tumors (5). In addition, age and consumption of alcohol and tobacco may be associated with newly formed malignant tumors after liver transplantation (6,7). Solid tumors are the main cause of liver transplantation-related mortality. It has been reported that the incidence of GC in liver transplant recipients is also increased, especially in Asian, where liver disease and GC pose great threat to public health (8-11).
Radical resection remains the major treatment for GC after liver transplantation. Gong et al. (12) reported that 19 of 2968 liver transplantation recipients were diagnosed with GC, and ESD was performed on 10 patients. Surgical resection was carried out on the remaining 8 patients as an initial treatment. One patient received chemotherapy first due to the peritoneal seeding revealed by preoperative CT scan. Until now, only a few cases of GC after liver transplantation have been reported (11,13,14). As presented in Table 1, Lee et al. (13) conducted an operation of laparoscopic-assisted radical gastrectomy for GC in 2011, and the others reported that laparotomy was performed. Compared with the surgery of primary GC, the operation of GC after liver transplantation is more difficult. The main reason is that the anatomical structure of upper abdomen has remarkably changed after liver transplantation: (I) extensive adhesions are formed among the organs and between the organs and abdominal wall; (II) the blood vessels in the hilum of liver are disrupted including the right gastric artery, which is an essential blood vessel in the stomach. In addition, the common hepatic artery becomes local dense adhesion after losing its normal sheath; (III) due to the exposure of blood vessels in the hilum during liver transplantation, the lymphatic reflux in the suprapubic region is impaired, especially the lymphatic reflux was blocked in group 5, 8 and 12.
Table 1
| Authors | Year of publication | Country | Age/sex | LDLT malignancy | Treatment | Stage | Chemo-therapy |
|---|---|---|---|---|---|---|---|
| Nagata et al. (15) | 2007 | Japan | 57/F | 24 month | Open | − | None |
| Arslan et al. (16) | 2011 | Turkey | 51/M | 120 month | Open | IB | None |
| Lee et al. (13) | 2011 | Korea | 72/M | 180 month | LAP | IA | None |
| Shimizu et al. (8) | 2012 | USA | 60/M | 30 month | Open | IIA | None |
| Li et al. (17) | 2013 | China | 55/M | 108 month | Open | IV | None |
| Takehara et al. (18) | 2014 | Japan | 65/F | 96 month | Open | IIIA | S-1 |
| Xiao et al. (14) | 2016 | China | 41/M | 144 month | Open | IIIA | − |
| Zhen et al. (19) | 2016 | China | 68/F; 67/M | 43/57 month | Open | − | None/YES |
| Yang et al. (11) | 2016 | China | 63/M | 96 month | Open | IIIC | None |
| Zhang et al. (20) | 2017 | China | 64/F | 120 month | − | II | PF |
| Present 2 cases | 2018 | China | 63/M;64/M | 132/11 month | Robtic | IIIB/IIIA | SOX |
Compared with traditional laparotomy and laparoscopic surgery, robotic surgery system provides better visual field exposure/clarity and operation flexibility. It also allows us to perform the operation in narrow space, and improves the relaxation of abdominal adhesion (3,21-23). In 2003, Hashizume et al. (24) reported the first successful robotic GC surgery. Since 2010, our team has been focused on robot-assisted radical gastrectomy for GC. At present, >1,000 operations have been performed, including 20 cases on gastric remnant after partial gastrectomy. In this study, robotic surgical system was used to perform radical gastrectomy on two patients.
During the operation, it is difficult to separate the adhesion of porta hepatis precisely without damaging the liver surface, ascending colon and duodenum. Attention should be paid to protect the common bile duct and portal vein during the precise isolation using ultrasonic knife and electric hook. No anatomical alterations were observed in the fundus and greater curvature of stomach, so the dissection of left gastroepiploic vessels and lymph nodes in group 4sb were the same as routine operations. The lymph nodes of group 5 and 8 on the surface of the common hepatic artery were replaced by proliferated vascular tissue. In case 1, left gastric artery and vein were not found, but notably increased artery blood supply on the upper edge of the lesser curvature was observed, which could be the compensatory hypertrophic arteries after liver transplantation. Additionally, the patient suffered from fundus esophageal varices, which also increased the difficulty of lymph node dissection in groups 1, 3 and 7. The reconstruction of digestive tract was completed with the assistance of robot, which provided the flexibility by using robotic endoscopic joints.
In case 1, 52 lymph nodes were dissected, but only 19 were confirmed by pathologist, as the others were locally proliferated adipose tissue. In case 2, 44 lymph nodes were isolated, but only 21 were analyzed by pathologist after operation, and the number was much lower compared with primary GC. The reason could be reduction of lymph nodes in the abdominal, perihepatic, upper and lower pyloric lymph node groups after liver transplantation, which was replaced by locally proliferated adipose fibrous tissue.
Postoperative management was similar as primary GC. The effects of immunosuppressive agents on wound healing should be minimized. In order to prevent acute rejection without inhibiting the anti-tumor activity excessively, the concentration of immunosuppressive agents should be fixed during the operation to maintain normal immune activity (18). In case 2, immunosuppressive agents were continuously provided during the surgery at the original dosage.
Robot surgery system with flexible joints enables us to operate in narrow spaces, and it is broadly used in isolating the adhesions between liver and colon/duodenum and dissecting the adhesions formed by impaired common hepatic artery which loses its vascular sheath. This study provides novel insight on the development of GC surgery after liver transplantation using robotic system. However, we also need more cases and longer follow-up time to support the conclusion.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-1786
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1786). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coratti A, Annecchiarico M, Di Marino M, et al. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg 2013;37:2771-81. [Crossref] [PubMed]
- Caruso S, Franceschini F, Patriti A, et al. Robot-assisted laparoscopic gastrectomy for gastric cancer. World J Gastrointest Endosc 2017;9:1-11. [Crossref] [PubMed]
- Qian F, Liu J, Liu J, et al. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:546-50. [Application of robotic surgery to treat carcinoma in the remnant stomach]. [PubMed]
- Sérée O, Altieri M, Guillaume E, et al. Longterm Risk of Solid Organ De Novo Malignancies After Liver Transplantation: A French National Study on 11,226 Patients. Liver Transpl 2018;24:1425-36. [Crossref] [PubMed]
- Miyazaki T, Sato S, Kondo T, et al. National survey of de novo malignancy after solid organ transplantation in Japan. Surg Today 2018;48:618-24. [Crossref] [PubMed]
- Mukthinuthalapati PK, Gotur R, Ghabril M. Incidence, risk factors and outcomes of de novo malignancies post liver transplantation. World J Hepatol 2016;8:533-44. [Crossref] [PubMed]
- Zhou J, Hu Z, Zhang Q, et al. Spectrum of De Novo Cancers and Predictors in Liver Transplantation: Analysis of the Scientific Registry of Transplant Recipients Database. PloS One 2016;11:e0155179. [Crossref] [PubMed]
- Shimizu T, Hayashi M, Inoue Y, et al. A case of gastric cancer after living donor liver transplantation. Ann Transplant 2012;17:122-6. [Crossref] [PubMed]
- Yu S, Gao F, Yu J, et al. De novo cancers following liver transplantation: a single center experience in China. PLoS One 2019;14:e0220430. [Crossref] [PubMed]
- Jung DH, Hwang S, Song GW, et al. Survival Benefit of Early Cancer Detection Through Regular Endoscopic Screening for De Novo Gastric and Colorectal Cancers in Korean Liver Transplant Recipients. Transplant Proc 2016;48:145-51. [Crossref] [PubMed]
- Yang K, Zhu H, Chen CC, et al. Lessons Learned From a Case of Gastric Cancer After Liver Transplantation for Hepatocellular Carcinoma: A Case Report and Literatures Review. Medicine (Baltimore) 2016;95:e2666. [Crossref] [PubMed]
- Gong CS, Yoo MW, Kim BS, et al. De Novo Gastric Cancer After Liver Transplantation. Ann Transplant 2016;21:386-91. [Crossref] [PubMed]
- Lee MS, Kim EY, Lee JH, et al. Laparoscopy-assisted distal gastrectomy for gastric cancer after liver transplantation. J Korean Surg Soc 2011;80:S1-5. [Crossref] [PubMed]
- Xiao H, Bian J, Zhang L, et al. Gastric cancer following a liver transplantation for glycogen storage disease type Ia (von Gierke disease): A case report. Oncol Lett 2014;8:2803-5. [Crossref] [PubMed]
- Nagata Y, Eguchi S, Takatsuki M, et al. Experience of gastric cancer in a patient who had received a living-donor liver transplantation. Gastric Cancer 2007;10:187-90. [Crossref] [PubMed]
- Arslan C, Kilickap S, Yalcin S. Gastric cancer after cadaveric liver transplantation in a patient with autoimmune hepatitis: A case report and review of the literature. Turk J Gastroenterol 2011;22:73-6. [Crossref] [PubMed]
- Li X, Zhu J, Li G, et al. De novo neoplasms after liver transplantation: a clinical study. Chinese Journal of Hepatobiliary Surgery 2013;19:102-4.
- Takehara H, Tanabe K, Fujikuni N, et al. A case of gastric cancer following living donor liver transplantation. Hiroshima J Med Sci 2014;63:23-6. [PubMed]
- Zhen W, Shipeng L, Jianjun Z. De novo digestive system malignancy after liver transplantation: a single-center clinical analysis of cases. Practical Journal of Organ Transplantation 2016;4:37-9.
- Zhang J, Zhou S, Wang H, et al. Chemotherapy for De Novo Gastric Adenocarcinoma After Deceased Orthotopic Liver Transplantation: A Case Report. Transplant Proc 2017;49:178-80. [Crossref] [PubMed]
- Liu XX, Jiang ZW, Chen P, et al. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol 2013;19:6427-37. [Crossref] [PubMed]
- Procopiuc L, Tudor S, Manuc M, et al. Open vs robotic radical gastrectomy for locally advanced gastric cancer. Int J Med Robot 2016;12:502-8. [Crossref] [PubMed]
- Caruso S, Patriti A, Roviello F, et al. Robot-assisted laparoscopic vs open gastrectomy for gastric cancer: Systematic review and meta-analysis. World J Clin Oncol 2017;8:273-84. [Crossref] [PubMed]
- Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am 2003;83:1429-44. [Crossref] [PubMed]


