
Lymphocyte-monocyte ratio as a predictive marker for pathological complete response to neoadjuvant therapy in esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC) is one of the most aggressive malignancies and has poor survival rates. Globally, EC is the seventh most common type of cancer and the sixth leading cause of cancer-related deaths worldwide (1). Esophageal squamous cell carcinoma (ESCC) is the most common histologic subtype of EC in China, accounting for approximately 90% of all cases, which in contrary to the predominance of esophageal adenocarcinoma in the Western countries (1,2). Because most EC patients are diagnosed at a late stage, more than 50% of patients have unresectable or metastatic disease at the time of diagnosis (3). For the remaining patients, who make up less than 50% of all patients, radical surgery still remains the optimal treatment, but with surgery alone, the 5-year overall survival (OS) rate is only 25% (4). During the past two decades, accumulating evidence has demonstrated that preoperative neoadjuvant chemoradiotherapy (nCRT) can significantly improve survival compared to that of surgery alone (5-8). Therefore, surgery along with preoperative nCRT has become the standard treatment for resectable locally advanced EC patients (5,6). However, not everyone seems to benefit from preoperative treatment. To date, strong evidence has confirmed that patients who achieve a pathological complete response (pCR) after nCRT have a significantly prolonged OS than that in patients who achieve a pathologic partial response or no response (9-11). Several studies demonstrated that pathological nonresponders have even worse survival than those who receive surgery alone (12,13). Therefore, we can see that only patients who achieve a pCR after neoadjuvant therapy can truly benefit from additional preoperative treatment. Otherwise, for patients who did not achieve pCR, due to the delay in the operation and the toxicity of additional chemoradiotherapy, the prognosis may be even worse than that in patients who underwent surgery alone. Therefore, to choose the best treatment for each individual, it is crucial to identify patients who can achieve pCR before the initiation of preoperative neoadjuvant treatment. In past studies, some researchers have proposed methods to predict pathological response in EC or other solid malignancies, such as PET/CT (14,15) and some molecular markers (16,17). However, due to the expensiveness and complexity of these techniques, they are not widely applied in a clinical setting currently.
Inflammation is a recognized hallmark of cancer that substantially contributes to the development and progression of malignancies (18). The maintenance of the systemic inflammatory response has been consistently observed to confer poor prognosis in both early and advanced stage disease (19). A series of studies have demonstrated that several inflammation-based markers, such as the serum neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and systemic immune-inflammation index (SII), are closely associated with the treatment response and outcomes in EC (20-26) or other solid tumors (27-30). However, relatively few studies have investigated the value of these hematological markers in predicting pathological response after neoadjuvant therapy in solid malignancies (31-35), especially in EC (34,35). In addition, the prognostic value of these biomarkers in EC remains uncertain and need further validation.
Hence, this aim of current study was to determine whether these hematological markers could predict the pCR in locally advanced esophageal squamous cell carcinoma (LA-ESCC) patients receiving nCRT. In addition, the prognostic impact of these hematological indicators for OS was assessed. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2849).
Methods
Patients selection
Between February 2012 and December 2015, a total of 87 ESCC patients who were treated at the Shandong Cancer Hospital and Institute (Jinan, China) were retrospectively enrolled in this analysis. They all received nCRT before undergoing radical surgery. Based on the chest and abdomen enhanced computed tomography (CT), positron emission tomography (PET)-CT and endoscopic ultrasonography (EUS) that were performed before the preoperative treatments, all patients underwent clinical tumor–node–metastasis (TNM) staging according to the 7th edition of the American Joint Committee on Cancer (AJCC) guidelines. The inclusion criteria were as follows: (I) histologically confirmed ESCC; (II) clinical stage II or III; (III) a Karnofsky performance status (KPS) ≥70; (IV) no previous antitumor treatment; (V) no history of other malignancy or secondary primary tumor; and (VI) without chronic or acute infection, hematologic disease, or autoimmune disease. Using the hospital information system, we extracted the following baseline characteristics: age, gender, KPS, smoking history, drinking history, tumor location, differentiation, clinical tumor (cT) stage, clinical node (cN) stage, and clinical tumor stage.
Definition of hematological markers
All patients underwent routine blood examinations within a week before the initiation of nCRT. The peripheral monocyte count, neutrophil count, lymphocyte count and platelet count were recorded. The pretreatment LMR, NLR, PLR and SII were calculated with the following formulas: LMR = absolute lymphocyte count/monocyte count; NLR = absolute neutrophil count/lymphocyte count; PLR = absolute platelet count/lymphocyte count; and SII = absolute platelet count × absolute neutrophil count/lymphocyte count.
Treatment
Before surgery, all the patients in this study received platinum-based chemotherapy concurrent with radiation therapy. All radiation treatments were implemented by either three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). They were treated with conventional fractionation (1.8/2.0 Gy fractions once daily for 5 days/week) and got a total dose of 45–50.4 Gy administered over 25–28 fractions. Chemotherapy began on Day 1 concurrent with the initial radiation treatments, and the regimens included 2 cycles of 5-fluorouracil (500 mg/m2, Days 1–5, intravenously) and cisplatin (75 mg/m2, Day 1, intravenously). A cycle of the chemotherapy regimen was repeated every 3 weeks. All 87 patients underwent R0 resection within 6–8 weeks after nCRT. Of the 87 patients, 66 (75.9%) underwent the esophagectomy by the left thoracic approach and 21 (24.1%) underwent the esophagectomy by the right thoracic approach. The majority of patients, 77 (88.5%) of them, received two-field lymphadenectomy, while the remaining 10 (11.5%) received three-field dissection. After surgery, 3 patients developed small anastomotic fistula, which was recovered after active treatment. In addition, another patient developed pneumonia, which was cured after antibiotics, cough and sputum removal, atomization and inhalation of drugs and other treatment measures. Other patients did not have serious postoperative complications.
Pathological examination
Pathological response was assessed for the resected primary tumors after surgery, and all assessments were independently performed by two pathologists. pCR was defined as no evidence of carcinoma on the final pathologic examination of the resected esophagus or lymph nodes.
Follow-up
Patients were followed up every 3 months within 2 years after surgery, every 6 months within 3–5 years, and annually after 5 years. At current study, we analyzed OS as the end point of prognosis. The OS was calculated from the starting date of nCRT to the date of death from any cause or the date of the last follow-up. Follow-up data were obtained from patient medical records and telephone interview. The median follow-up period for the assessment of OS was 29 months (range, 9.6–77.4 months).
Statistical analyses
Statistical analysis was conducted with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as numbers and percentages. Laboratory variables, which recorded as continuous variables, were expressed as the means with standard deviations. Clinicopathological parameters were assessed using the chi-squared test, and continuous variables were compared using the Mann-Whitney U test. The optimal cut-off values of the LMR, NLR, PLR, and SII for predicting pCR were determined using a receiver operating characteristic (ROC) curve. Univariable and multivariable logistic regressions were performed to determine the predictors of pCR. The OS was analyzed using Kaplan-Meier plots and compared with the log-rank test. Variables for which the P value was <0.05 in the univariate analysis were included into a multivariable Cox proportional hazards model. When both the cT stage and clinical stage showed statistical significance in univariate analysis, in order to avoid mutual influence, we only included clinical stage in multivariable analysis. All tests were two sided, and P<0.05 was considered statistically significant.
Results
Patients characteristics
The patient’s baseline characteristics are listed in Table 1. A total of 87 LA-ESCC patients who received nCRT followed by radical esophagectomy were included in our analysis. The patient cohort included 73 (83.9%) males and 14 (16.1%) females, and the mean age was 57.69±7.34 years (range, 39–75 years). Among the 87 cases, ESCC in the upper-thoracic, middle-thoracic and lower-thoracic regions accounted for 12.6%, 54.0% and 33.3%, respectively, of the cases. The cT stage was T2 in 8 patients (9.2%), T3 in 67 patients (77.0%) and T4 in 12 patients (13.8%). In 64 (73.6%) patients, the nodal status was positive. Patients in clinical stage II or III accounted for 25 (28.7%) and 62 (71.3%) of the cases, respectively. After nCRT, 26 (29.9%) patients achieved pCR, while 61 (70.1%) did not.
Table 1
| Variables | All patients (n=87) | pCR (n=26) | Non-pCR (n=61) | P |
|---|---|---|---|---|
| Age (years)a | 57.69±7.34 | 55.73±7.74 | 58.52±7.06 | 0.084 |
| Gender | 0.247 | |||
| Male | 73 (83.9%) | 20 (76.9%) | 53 (86.9%) | |
| Female | 14 (16.1%) | 6 (23.1%) | 8 (13.1%) | |
| KPS | 0.907 | |||
| <80 | 14 (16.1%) | 4 (15.4%) | 10 (16.4%) | |
| ≥80 | 73 (83.9%) | 22 (84.6%) | 51 (83.6%) | |
| Smoking | 0.429 | |||
| Never | 25 (28.7%) | 9 (34.6%) | 16 (26.2%) | |
| Ever | 62 (71.3%) | 17 (65.4) | 45 (73.8%) | |
| Drinking | 0.259 | |||
| Never | 23 (26.4%) | 9 (34.6%) | 14 (23.0%) | |
| Ever | 64 (73.6%) | 17 (65.4%) | 47 (77.0%) | |
| Tumor location | 0.840 | |||
| Upper | 11 (12.6%) | 4 (15.4%) | 7 (11.5%) | |
| Middle | 47 (54.0%) | 13 (50.0%) | 34 (55.7%) | |
| Lower | 29 (33.3%) | 9 (34.6%) | 20 (32.8%) | |
| Differentiation | 0.255 | |||
| Well or moderate | 63 (72.4%) | 21 (80.8%) | 42 (68.9%) | |
| Poor | 24 (27.6%) | 5 (19.2%) | 19 (31.1%) | |
| T stage | 0.035* | |||
| T2 | 8 (9.2%) | 5 (19.2%) | 3 (4.9%) | |
| T3 | 67 (77.0%) | 20 (76.9%) | 47 (77.0%) | |
| T4 | 12 (13.8%) | 1 (3.8%) | 11 (18.0%) | |
| N stage | 0.946 | |||
| N0 | 23 (26.4%) | 7 (26.9%) | 16 (26.2%) | |
| N+ | 64 (73.6%) | 19 (73.1%) | 45 (73.8%) | |
| Tumor stage | 0.019* | |||
| II | 25 (28.7%) | 12 (46.2%) | 13 (21.3%) | |
| III | 62 (71.3%) | 14 (53.8%) | 48 (78.7%) | |
| LMRa | 3.63±1.39 | 4.35±1.68 | 3.33±1.13 | 0.002* |
| NLRa | 2.95±1.72 | 2.51±1.16 | 3.14±1.89 | 0.143 |
| PLRa | 157.3±53.5 | 140.7±52.1 | 164.4±52.9 | 0.068 |
| SIIa | 741.9±506.0 | 564.9±272.2 | 817.3±562.8 | 0.032* |
a, data presented as mean ± standard deviation; *, P<0.05 was considered significant. pCR, pathological complete response; KPS, Karnofsky performance status; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SII, systemic immune-inflammation index.
Table 1 also showed the findings of the comparison that was performed between pCR and non-pCR among the 87 cases. As shown in Table 1, the LMR was significantly higher in patients who achieved pCR compared to that in patients who did not achieve pCR (4.35±1.68 vs. 3.33±1.13, P=0.002). In addition, the SII was significantly lower in patients who achieved pCR compared to that in patients who did not achieve pCR (564.9±272.2 vs. 817.3±562.8, P=0.032). The cT stage and clinical tumor stage were also significantly correlated with pCR (P=0.035, 0.019, respectively).
Analysis of ROC curves
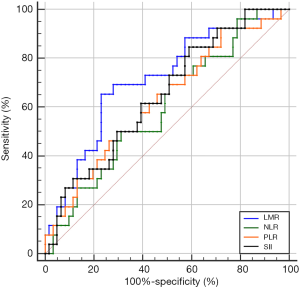
The optimal cutoff values of LMR, NLR, PLR, and SII for predicting pCR were 3.73 (sensitivity 65.4%, specificity 77%), 2.92 (sensitivity 49.2%, specificity 73.1%), 130.89 (sensitivity 70.5%, specificity 50%), and 792.49 (sensitivity 41%, specificity 84.6%), respectively. The area under the ROC curves (AUCs) were 0.712 [95% confidence interval (CI): 0.594–0.830; P=0.002], 0.600 (95% CI: 0.473–0.726; P=0.143), 0.626 (95% CI: 0.498–0.754; P=0.064), and 0.646 (95% CI: 0.524–0.768; P=0.032) for LMR, NLR, PLR, and SII, accordingly (Figure 1). Patients were then divided into high or low groups: high-LMR group (≥3.73, n=29), low-LMR group (<3.73, n=58); high-NLR group (≥2.92, n=38), low-NLR group (<2.92, n=49); high-PLR group (≥130.89, n=56), low-PLR group (<130.89, n=31); high-SII group (≥792.49, n=32) and low-SII group (<792.49, n=55).
Comparison of pCR rates between high and low groups
By chi-squared test, we found that the pCR rate was significantly higher in the high-LMR group than in the low-LMR group (53.1% vs. 16.4%, P<0.001). Meanwhile, compared with patients who had a high SII, the low-SII group had a significantly higher rate of pCR (37.9% vs. 13.8%, P=0.020). However, there was no significant difference in pCR rates between the different NLR or PLR groups (P=0.113, P=0.068, respectively).
Univariate and multivariate analyses of the variables predicting pCR
In the univariate analysis (Table 2), in addition to LMR (P=0.001) and SII (P=0.026), the age (P=0.044), cT stage (P=0.017) and clinical tumor stage (P=0.022) were also significantly associated with pCR. In the multivariate analysis (Table 2), the age [OR (odd ratio): 0.291; 95% CI: 0.090–0.938; P=0.039], clinical tumor stage (OR: 0.258; 95% CI: 0.080–0.831; P=0.023) and LMR (OR: 5.093; 95% CI: 1.658–15.646; P=0.004) remained as independent predictors of pCR.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | ||
| Age (<60/≥60 years) | 0.357 | 0.131–0.971 | 0.044* | 0.291 | 0.090–0.938 | 0.039* | |
| Gender (male/female) | 1.987 | 0.613–6.448 | 0.253 | ||||
| KPS (<80/≥80) | 1.078 | 0.305–3.812 | 0.907 | ||||
| Smoking (never/ever) | 0.672 | 0.250–1.806 | 0.430 | ||||
| Drinking (never/ever) | 0.563 | 0.206–1.536 | 0.262 | ||||
| Tumor location (upper /middle /lower) | 0.951 | 0.468–1.935 | 0.891 | ||||
| Differentiation (well or moderate/poor) | 0.526 | 0.172–1.606 | 0.260 | ||||
| cT stage (T2/T3/T4) | 0.239 | 0.074–0.772 | 0.017* | ||||
| cN stage (N0/N+) | 0.965 | 0.342–2.724 | 0.946 | ||||
| Tumor stage (II/III) | 0.316 | 0.118–0.846 | 0.022* | 0.258 | 0.080–0.831 | 0.023* | |
| LMR (<3.73/≥3.73) | 5.793 | 2.139–15.686 | 0.001* | 5.093 | 1.658–15.646 | 0.004* | |
| NLR (<2.92/≥2.92) | 0.459 | 0.174–1.214 | 0.117 | ||||
| PLR (<130.89/≥130.89) | 0.419 | 0.163–1.077 | 0.071 | ||||
| SII (<792.49/≥792.49) | 0.262 | 0.080–0.853 | 0.026* | 0.644 | 0.168–2.476 | 0.522 | |
*, P<0.05 was considered significant. pCR, pathological complete response; KPS, Karnofsky performance status; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SII, systemic immune-inflammation index; OR, odds ratio; CI, confidence interval.
Prognostic variables for OS
For the entire cohort, the median OS was 26 months (range, 9.6–72.1 months). As shown in Kaplan-Meier analysis, compared to their matched counterparts, the patients with high LMR (median OS: 38.0 vs. 21.2 months, P<0.001), low NLR (median OS: 33.3 vs. 18.6 months, P=0.039) and SII (median OS: 34.1 vs. 16.8 months, P=0.001) was associated with prolonged OS (Figure 2). Furthermore, the patients obtained pCR after neoadjuvant therapy also showed the longer OS than those who did not (median OS: 43.5 vs. 25.4 months, P<0.001, Figure 3). In univariate analysis, the cT stage (P=0.001), clinical stage (P=0.007), pCR (P<0.001), lymph node dissection mode (P=0.040), LMR (P<0.001), NLR (P=0.041) and SII (P=0.001) were identified as the significant prognostic factors (Table 3). However, the results of multivariate analysis revealed that only clinical stage [HR (hazard ratio): 1.970; 95% CI: 1.144–3.391; P=0.014], pCR (HR: 0.469; 95% CI: 0.237–0.928, P=0.030) were independent prognostic factors for OS (Table 3). None of the inflammatory biomarkers (LMR, NLR, PLR, and SII) can be used as independent predictors of OS.
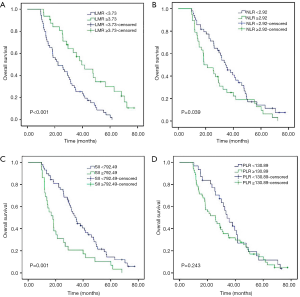
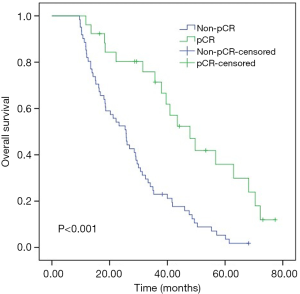
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age(years) (<60/≥60) | 1.479 | 0.935–2.339 | 0.094 | ||||
| Gender (male/female) | 0.863 | 0.443–1.683 | 0.666 | ||||
| KPS (<80/≥80) | 0.767 | 0.422–1.396 | 0.386 | ||||
| Smoking (never/ever) | 1.435 | 0.851–2.420 | 0.175 | ||||
| Drinking (never/ever) | 1.522 | 0.894–2.592 | 0.122 | ||||
| Tumor location (upper/middle/lower) | 1.141 | 0.807–1.614 | 0.455 | ||||
| Differentiation (well or moderate/poor) | 1.026 | 0.620–1.697 | 0.920 | ||||
| cT stage (T2/T3/T4) | 2.529 | 1.475–4.338 | 0.001* | ||||
| cN stage (N0/N+) | 1.247 | 0.732–2.123 | 0.417 | ||||
| Tumor stage (II/III) | 2.082 | 1.223–3.552 | 0.007* | 1.970 | 1.144–3.391 | 0.014* | |
| Pathological response (pCR/non-pCR) | 0.319 | 0.180–0.564 | <0.001* | 0.469 | 0.237–0.928 | 0.030* | |
| Operation approach (left/right thoracic) | 0.912 | 0.542–1.537 | 0.730 | ||||
| Lymph node dissection mode (two/three-field) | 0.459 | 0.219–0.964 | 0.040* | 0.658 | 0.302–1.432 | 0.291 | |
| LMR (<3.73/≥3.73) | 0.378 | 0.223–0.639 | <0.001* | 0.640 | 0.335–1.221 | 0.175 | |
| NLR (<2.92/≥2.92) | 1.601 | 1.020–2.513 | 0.041* | 0.896 | 0.518–1.550 | 0.694 | |
| PLR (<130.89/≥130.89) | 1.322 | 0.825–2.121 | 0.245 | ||||
| SII (<792.49/≥792.49) | 2.226 | 1.391–3.562 | 0.001* | 1.535 | 0.866–2.720 | 0.142 | |
*, P<0.05 was considered significant. OS, overall survival; KPS, Karnofsky performance status; pCR, pathological complete response; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SII, systemic immune-inflammation index; HR, hazard ratio; CI, confidence interval.
Discussion
In present study, we retrospectively analyzed a consecutive cohort of LA-ESCC patients who underwent nCRT and found that LMR is an independent predictive marker of pCR. Moreover, the patients achieving a pCR have a significantly prolonged OS. To the best of our knowledge, this is the first report that focused on the role of LMR in predicting pCR in ESCC.
Preoperative nCRT followed by radical esophagectomy has increasingly been used as the standard treatment pattern for locally advanced EC (5,6). Many studies have reached the same conclusion that patients who achieve pCR after neoadjuvant therapy have prolonged OS compared than those who did not achieve pCR in EC (9-11). Ancona et al. even demonstrated that only achieving pCR can significantly improve the survival of patients with resectable ESCC, and the survival of patients who had a pathologic partial response or no response was comparable to that of patients who underwent surgery alone (36). In other words, only patients who respond completely to neoadjuvant therapy can benefit from additional preoperative treatment. Therefore, early prediction of pCR is crucial for the development of individualized treatment strategies.
In the past decade, it has become clear that the maintenance of the systemic inflammatory response is associated with poor outcomes in many solid malignancies (19). Recently, systemic inflammatory markers, including LMR, NLR, PLR and SII, have been proved to be closely associated with the treatment response and prognosis in a variety of cancers, including ESCC (20-30).
The relationships between NLR, PLR and pathological response to neoadjuvant therapy have been investigated in some solid tumors (31-33). A recent meta-analysis demonstrated that a lower NLR was associated with a greater chance for pCR and may predict better survival for patients with solid tumors (31). Additionally, Hasegawa et al. reported that an elevated NLR is an independent risk factor for a poor pathological response in patients with pancreatic cancer (32). In HER2-negative breast cancer, Hu et al. reported that a low pretreatment PLR was related to higher pCR rates and was an independent prognostic factor for better disease-free survival (DFS) (33). Few studies have demonstrated that NLR, PLR can be used to predict the pathological response of preoperative treatment in patients with EC (34,35). McLaren et al. demonstrated that elevated NLR and PLR were both negative predictors of pCR in patients with EC (34). Similarly, Sato et al. also demonstrated that pretreatment elevated NLR was a significant predictive marker for a poor pathologic response in EC (35). However, our study showed that NLR and PLR were not significantly associated with pCR based on the univariate analysis, which is not consistent with McLaren or Sato’s conclusions (34,35). This variability may be due to the different standards for the inclusion of patients, different surgical timing or the heterogeneity of the patients and tumors themselves.
The SII, an integrated indicator based on the peripheral lymphocyte, neutrophil, and platelet counts, was first developed by Hu et al. (37), and it has been demonstrated that an elevated SII is associated with poor prognosis in hepatocellular carcinoma. In EC, the prognostic value of SII has also been widely investigated (23-25). A recent meta-analysis of five original studies showed that SII was a promising predictor of survival in EC. The higher the SII before treatment, the worse the OS (23). In addition, two other studies confirmed in surgically resected ESCC that SII was an independent prognostic factor. The high preoperative SII was an indicator of aggressive biology and worse prognosis (24,25). However, few studies have investigated the predictive effect of SII on pCR in EC patients who underwent neoadjuvant therapy plus surgery. In present study, we evaluated the value of pretreatment SII in predicting pCR and OS. The results showed that low SII was associated with pCR and prolonged OS in univariate analysis, although multivariate analysis did not prove its independent predictive value.
Moreover, increasing evidence has identified that an elevated LMR can predict a favorable treatment response and better prognosis in solid tumor (28-30). A recent meta-analysis of head and neck cancer reported that an elevated LMR may be an indicator of a favorable prognosis (28). Zhu et al. found that in patients with advanced epithelial ovarian cancer, the low-LMR group was associated with poor chemotherapy sensitivity, and low-LMR was an independent adverse prognostic factor for PFS and OS (29). In locally advanced breast cancer, Ni et al. have shown that an elevated LMR can predict a favorable treatment response and favorable prognosis in patients treated with neoadjuvant chemotherapy followed by surgery (30). In studies about ESCC, Huang et al. retrospectively analyzed data from 348 patients who underwent radical surgery and found that patients with a low LMR had a significantly worse 5-year cancer-specific survival than that of patients with a high LMR (20). Additionally, Song et al. reported that low preoperative LMR could serve as an independent prognostic factor of poor DFS and OS in ESCC patients undergoing curative surgery (21). Liu et al. reported that patients with a high LMR showed a good clinical tumor response and that high LMR was an independent prognostic for longer PFS and OS in ESCC patients treated with definitive chemoradiotherapy (22). Unfortunately, the three studies described above all focused on the relationships between the LMR and long-term survival of patients who received radical surgery or definitive chemoradiotherapy, and none of the studies focused on the efficacy of neoadjuvant therapy (20-22). In present study, we analyzed the correlation between the LMR and the therapeutic effect of neoadjuvant therapy, as evaluated by the pathological response of 87 ESCC patients who received nCRT. The results of present study showed that the pCR rate was significantly higher in patients with a high LMR. Meanwhile, based on multivariate analyses, we demonstrated that the LMR is an independent predictive factor of pCR. This suggested that before administering nCRT to ESCC patients, clinicians could use the LMR to predict the likelihood of achieving pCR. For patients with an elevated LMR before treatment, the possibility of obtaining pCR is high, and the neoadjuvant therapy should be given more actively.
However, the mechanism of the predictive value of the LMR for pCR have not yet been clarified. There are some hypotheses on this issue. A higher LMR indicated a relatively high number of lymphocytes and a relatively low number of monocytes. Firstly, lymphocytes play a critical role in promoting the antitumor immune response through immunologic surveillance and tumor immunoediting. They can induce tumor cell apoptosis, and then inhibit the tumor cell proliferation and migration (38). Among them, CD8+ T cells are the dominating effector cells, responsible for killing cancer cells and eradicating the tumor (39). Tumor-infiltrating T cells (TILs) that evolved from circulating lymphocytes into the tumor microenvironment have been shown to be associated with better pathological response to neoadjuvant chemotherapy and prolonged cancer-specific survival in EC (40). Moreover, a previous study of EC directly confirmed that a higher circulating lymphocyte count during nCRT is associated with a higher pCR rate (41). Secondly, in contrast to the antitumor effect exerted by lymphocytes, monocytes appear to play the opposite role. Circulating monocytes can be recruited into the tumor microenvironment and differentiate into tumor-associated macrophages (TAMs) under the influence of growth factors and chemokines produced by tumor cells and tumor stromal cells (42). TAMs can generate a series of growth factors or cytokines, such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), interleukin (IL)-6, matrix metalloproteinases (MMPs), thereby promoting tumor proliferation, angiogenesis and lymphangiogenesis, inhibiting specific antitumor immunity, reshaping extracellular matrix, which ultimately promote tumor progression and metastasis (43). Shibutani et al. reported that the peripheral monocyte count was associated with the density of the TAMs (44). Therefore, we can assume that the peripheral levels of monocytes could be an indirect and easily accessible surrogate for TAMs existing in the tumor microenvironment. In short, LMR reflects the balance between antitumor immune response and pro-tumor inflammatory response. Therefore, a high LMR represents a strong antitumor immune response and a weakened inflammatory response. This may be why pre-treatment high LMR can predict the pCR in ESCC patients receiving nCRT.
Our research has many limitations. A major limitation is the retrospective nature, and the number of patients is relatively low. Consequently, there are potential confounding factors that we cannot control. Therefore, additional prospective studies involving multiple centers are needed to confirm the reproducibility of our results and to explore these effects more comprehensively. Furthermore, since the primary endpoint of our study was pathological response after neoadjuvant therapy, in terms of survival, we only used OS as the secondary endpoint, but did not study relapse-free survival. This is another limitation of our study.
Conclusions
In LA-ESCC, the patients with pCR after nCRT live longer. In our patient population, having pretreatment elevated LMR suggested a high likelihood of pCR to neoadjuvant treatment. The pretreatment LMR therefore can be used as an early predictor of pCR, thereby promoting individualized treatment strategies for each LA-ESCC patient.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2849
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2849
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2849). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee of Shandong Cancer Hospital and Institute approved the study (No.201806015). All participants signed informed consent forms. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Wong I, Law S. The CROSS road in neoadjuvant therapy for esophageal cancer: long-term results of CROSS trial. Transl Cancer Res 2016;5:S415-S9. [Crossref]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. [Crossref] [PubMed]
- Alnaji RM, Du W, Gabriel E, et al. Pathologic Complete Response Is an Independent Predictor of Improved Survival Following Neoadjuvant Chemoradiation for Esophageal Adenocarcinoma. J Gastrointest Surg 2016;20:1541-6. [Crossref] [PubMed]
- Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 2017;123:4106-13. [Crossref] [PubMed]
- Dittrick GW, Weber JM, Shridhar R, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol 2012;19:1678-84. [Crossref] [PubMed]
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [Crossref] [PubMed]
- Borggreve AS, Goense L, van Rossum PSN, et al. Preoperative Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients With Esophageal Cancer Using (18)F-FDG PET/CT and DW-MRI: A Prospective Multicenter Study. Int J Radiat Oncol Biol Phys 2020;106:998-1009. [Crossref] [PubMed]
- Harada K, Wang X, Shimodaira Y, et al. Early Metabolic Change after Induction Chemotherapy Predicts Histologic Response and Prognosis in Patients with Esophageal Cancer: Secondary Analysis of a Randomized Trial. Target Oncol 2018;13:99-106. [Crossref] [PubMed]
- Nakanoko T, Saeki H, Morita M, et al. Rad51 expression is a useful predictive factor for the efficacy of neoadjuvant chemoradiotherapy in squamous cell carcinoma of the esophagus. Ann Surg Oncol 2014;21:597-604. [Crossref] [PubMed]
- Zhou S, Zhao L, Liang Z, et al. Indoleamine 2,3-dioxygenase 1 and Programmed Cell Death-ligand 1 Co-expression Predicts Poor Pathologic Response and Recurrence in Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemoradiotherapy. Cancers (Basel) 2019;11:169. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 2014;110:1409-12. [Crossref] [PubMed]
- Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 2015;8:137-45. [PubMed]
- Song Q, Wu JZ, Wang S. Low Preoperative Lymphocyte to Monocyte Ratio Serves as a Worse Prognostic Marker in Patients with Esophageal Squamous Cell Carcinoma Undergoing Curative Tumor Resection. J Cancer 2019;10:2057-62. [Crossref] [PubMed]
- Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. Onco Targets Ther 2017;10:871-7. [Crossref] [PubMed]
- Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res 2019;11:4185-200. [Crossref] [PubMed]
- Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer 2019;10:3188-96. [Crossref] [PubMed]
- Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 2019;234:1794-802. [Crossref] [PubMed]
- Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil: lymphocyte ratio is superior to the platelet: lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2016;29:403-11. [Crossref] [PubMed]
- Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261-72. [Crossref] [PubMed]
- Tham T, Olson C, Khaymovich J, et al. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2018;275:1663-70. [Crossref] [PubMed]
- Zhu JY, Liu CC, Wang L, et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer 2017;8:737-43. [Crossref] [PubMed]
- Ni XJ, Zhang XL, Ou-Yang QW, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One 2014;9:e111886. [Crossref] [PubMed]
- Li X, Dai D, Chen B, et al. The value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: A systematic review and meta-analysis. J Cancer 2018;9:861-71. [Crossref] [PubMed]
- Hasegawa S, Eguchi H, Tomokuni A, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett 2016;11:1560-6. [Crossref] [PubMed]
- Hu Y, Wang S, Ding N, et al. Platelet/Lymphocyte Ratio Is Superior to Neutrophil/Lymphocyte Ratio as a Predictor of Chemotherapy Response and Disease-free Survival in Luminal B-like (HER2(-)) Breast Cancer. Clin Breast Cancer 2020. [Epub ahead of print].
- McLaren PJ, Bronson NW, Hart KD, et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios can Predict Treatment Response to Neoadjuvant Therapy in Esophageal Cancer. J Gastrointest Surg 2017;21:607-13. [Crossref] [PubMed]
- Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617-22. [Crossref] [PubMed]
- Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91:2165-74. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Nazir T, Islam A, Omer MO, et al. Lymphocytopenia; induced by vinorelbine, doxorubicin and cisplatin in human cancer patients. Breast Dis 2015;35:1-4. [Crossref] [PubMed]
- Martinez-Lostao L, Anel A, Pardo J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res 2015;21:5047-56. [Crossref] [PubMed]
- Noble F, Mellows T, McCormick Matthews LH, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother 2016;65:651-62. [Crossref] [PubMed]
- Fang P, Jiang W, Davuluri R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol 2018;128:584-90. [Crossref] [PubMed]
- Galdiero MR, Marone G, Mantovani A. Cancer Inflammation and Cytokines. Cold Spring Harb Perspect Biol 2018;10. [PubMed]
- Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal 2014;2014:521754.
- Shibutani M, Maeda K, Nagahara H, et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer 2017;17:404. [Crossref] [PubMed]


