
The overexpression of miRNA-212-5p inhibited the malignant proliferation of liver cancer cells HepG2 and the tumor formation in nude mice with transplanted tumor through down-regulating SOCS5
Introduction
Liver cancer is the fifth most common cancer in the world, and the third leading cause of cancer death worldwide (1). Despite recent progress in disease diagnosis and treatment, the long-term prognosis of patients with liver cancer is still poor. For patients with advanced liver cancer, the overall 5-year survival rate is less than 5% (2). The main challenge of liver cancer treatment is recurrence and metastasis in the liver, which leads to a poor prognosis of liver cancer patients (3). MicroRNA is a class of non-coding RNA of 20 to 22 nucleotides, which can effectively regulate various biological processes, including cell proliferation, migration, differentiation, and apoptosis (4). The uncontrolled expression of microRNA is closely related to the pathogenesis of cancer, and it may act as a tumor suppressor gene or oncogene to promote the occurrence and development of liver cancer (5). Therefore, further research on the expression pattern and the role of microRNA may supply a new diagnostic and therapeutic target for liver cancer. miR-212 is a new miRNA associated with cancer, and it has been found that miR-212 is involved in the progress of different types of human cancers and down-regulated in liver cancer tissues (6). Besides, some studies have shown that the up-regulation of miR-212 inhibits the migration and tumorigenicity of human hepatocellular carcinoma (7). miR-212 is a potential prognostic biomarker of hepatocellular carcinoma, overexpression of miR-212 inhibits the migration of hepatocellular carcinoma cells in vitro and in vivo (8). Hepatitis B virus triggers apolipoprotein B mRNA editing enzyme catalytic subunit 2 (APOBEC2) expression through miR 122 regulation and affects the proliferation of liver cancer cells (9). miR-212 inhibits the growth of human liver cancer tumors by targeting FOXA1 (10). A study has exhibited that aberrant hypermethylation-induced downregulation of miR-212-3p results in overexpression of Mucin 13 in intrahepatic cholangiocarcinoma, leading to metastasis via activation of the EGFR/PI3K/AKT signaling pathway (11).
Suppressor of cytokine signaling 5 (SOCS5) is a member of inhibitors of the cytokine signaling protein family, and it has been shown to have a tumor-suppressive effect on liver cancer recently (12). SOCS5 was significantly overexpressed in hepatocellular carcinoma tissues, compared to adjacent non-tumor liver tissues. SOCS5 overexpression promoted cell migration and invasion in vitro by inactivating PI3K/Akt/mTOR-mediated autophagy (13). SOCS5 contributes to tumorigenesis, SOCS5/miR-18a/miR-25 axis regulates tumor suppressor TSC1 and downstream mTOR signaling in liver cancer (14). Importantly, HAND2-AS1 enhanced inactivation of the JAK-STAT pathway through sponging miR-3118 and facilitating SOCS5 to retard cell proliferation and migration in liver cancer (15). This article aims to investigate the effect of miR-212-5p overexpression targeting SOCS5 on the malignant proliferation of hepatocellular carcinoma HepG2 and tumor formation in nude mice. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2007).
Methods
Experimental reagent
Roswell Park Memorial Institute 1640 (RPMI 1640) medium (61870-127), fetal bovine serum (26400-036), penicillin-streptomycin (15140-122), and trypsin (25200-056) were purchased from Gibco, USA. The luciferase (L7840) detection kit was purchased from solarbio. The BCA protein concentration determination kit (P0012S), Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (C1062S), and terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL) apoptosis detection kit (C1091) were bought from Shanghai Biyuntian Biotechnology Institute. The Anti Ki67 (ab15580), proliferating cell nuclear antigen (PCNA) (ab18197), Bax (ab53154), Bcl-2 (ab196495), Caspase-3 (ab13847), cleaved caspase-3 (ab2302), Caspase-9 (ab52298), cleaved Caspase-9 (ab2324), phosphatidylinositol 3 kinase (PI3K) (ab191606), p-PI3K (ab182651), protein kinase B (AKT) (ab106693), p-AKT (ab192623), mTOR (ab2732), and β-Actin (ab8227) were purchased from Abcam, USA.
Cell culture
The human liver cancer cell line HepG2 was derived from the Chinese Type Culture Collection, which was cultured in RPMI 1640 medium (containing 10% fetal bovine serum, 1×105 U/L penicillin and 1×105 U/L streptomycin), and then incubated in an incubator with 5% carbon dioxide at 37 °C. The medium was changed every two days, and the cells were passaged on average between 3 and 4 days. The cells at logarithmic growth phase were used in this experiment.
RT-PCR
First, the total RNA was extracted from nude mouse tumor tissues and HepG2 cells by the Trizol method, and then the absorbance was measured with Nanodrop spectrophotometer (A260/A280). After that, the cDNA synthesis and PCR amplification were performed according to the kit instructions. The reaction conditions were set to 94 °C pre denatured for 5 minutes, then denatured at 94 °C for 30 seconds, annealed at 55 °C for 30 seconds, extended at 72 °C for 30 seconds, and amplified for 35 cycles. Afterward, it was extended at 72 °C for 10 minutes and stored at 4 °C. The reaction products were separated by 2% agarose gel electrophoresis. The qRT-PCR data were analyzed using 2–ΔΔCt method to calculate the relative expression levels of mRNA.
Luciferase report experiment
Luciferase reporter analysis was used to detect whether SOCS5 is the direct downstream target gene of miR-212-5p. First, wild-type and mutant SOCS5 3’UTR luciferase reporter gene plasmids were constructed, which were then washed with PBS. After that, the washing solution was poured off, 1× ULB was added, and mixed for 5 minutes in a micro-shaker. kit Twenty µL of sample and 100 µL Reagent I was added to the bottom of the measuring tube according to the instructions of the, tapping the tube wall 3–5 times to mix. Then, the tube was put into the instrument and measured at once, and the luminescence value was recorded as the luminescence unit (RLU) of Firefly luciferase. After that, 100 µL Rassay Reagent II was at once added to the bottom of the tube, gently tapping the tube wall for 3 to 5 times to mix, which was then immediately measured in the instrument. Finally, the luminescence value was recorded as the luminescence unit (RLU) of Ranilla luciferase, which serves as an internal reference.
SOCS5 overexpression
The SOCS5 pcDNA vector was constructed to overexpress SOCS5 and transfected into HepG2 cells. The cells were randomly divided into three groups, the control group, the pcDNA group, and the pcDNA-SOCS5 group. RT-PCR and Western blot were used to determine whether the overexpression was successful.
Cell grouping
miR-212-5p mimic and pcDNA-SOCS5 were transfected into HepG2 cells alone or in combination, and the cells were randomly divided into four groups, the control group, the mimic group, the SOCS5 group, and the mimic + SOCS5 group.
Clone formation method
First, HepG2 cells were cultivated to about 30% confluence in a petri dish. Then, the cells were cultured for another four days and blew up into single cells. After that, the cell was cultured in a 6-well plate with 500 cells per well for 14 days. After that, the medium was discarded, and the cell was fixed with ethanol for 30 minutes and stained with 0.5% crystal violet. Finally, the cell was rinsed with deionized water to dry, and the photo was taken for observation.
Flow cytometry to detect apoptosis
First, the suspended cells were collected into a 10 mL centrifuge tube, with 3×106/mL cells per sample, centrifuged at 500–1,000 r/minutes for 5 minutes, and the culture solution was discarded. Then, the cells were washed once with incubation buffer, and centrifuged at 500–1,000 r/minutes for 5 minutes, which was then suspended with 100 ul of labeling solution, and incubated at room temperature in the dark for 10–15 minutes. After that, the cells were centrifuged at 500–1,000 r/minutes for 5 minutes and washed once with the cell incubation buffer. Then, the fluorescent (SA-FLOUS) solution was added, and incubated at 4 °C for 20 minutes, avoiding light and vibrating occasionally. Finally, flow cytometry analysis was performed. The flow cytometry excitation wavelength was 488nm, a bandpass filter with a wavelength of 515 nm was used to detect FITC fluorescence, and another filter with a wavelength greater than 560 nm was used to detect PI.
Nude mouse subcutaneous xenograft model
All animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Second Affiliated Hospital of Air Force Medical University. Ten Balb/c nude mice were bought from the Animal Experiment Center of Zhejiang University of Traditional Chinese Medicine, male, 4–6 weeks old, weighing 16±2 g. The license number was SCXK (Zhejiang) 2018-0003. The untreated HepG2 cells in the logarithmic growth phase and HepG2 cells transfected with miR-212-5p mimic were suspended in medium, and the cell concentration was adjusted to 1×107/mL. The skin of nude mice was disinfected with 75% alcohol, and then 0.2 mL of cell suspension was injected subcutaneously in the right armpit of nude mice. Tumor growth can be seen 14 days after inoculation. When the tumor volume grows to 80–100 mm3, the nude mice bearing tumors are randomly divided into two groups, the control group, and the pc-Nrdp1 group.
Tumor volume
After the model was set up, the long and short diameters of the tumors were recorded every five days from day 0, volume = long diameter × short diameter2 ×1/2. After recording the long and short diameters of the transplanted tumor for 30 days, the nude mice were sacrificed by the neck-breaking method.
Immunohistochemical staining
Tumor tissues of each group were taken and embedded in conventional paraffin to make slices with a thickness of about 4 µm. Immunohistochemical staining was carried out according to the instructions of the immunohistochemical detection kit., and the DAB chromogenic kit was used for color development, hematoxylin for counterstaining, hydrochloric acid alcohol for color separation, and the expression of Ki67 in tumor tissue was observed by light microscope.
TUNEL
Part of the tumor tissue was used to make paraffin sections and routinely dewaxed to water, which was then immersed in the 0.1 M citric acid buffer with pH =6.0, treated in the microwave for 5 minutes, and washed twice with PBS. After that, the section was added in the wet box with a TUNEL reaction solution for the dark reaction at 37 °C for one hour and washed with PBS 3 times. POD was added to the slices for reacting at 37 °C for 30 minutes in a wet box and washed three times with PBS after the slides were dried. Then, DAB was added to react for 10 minutes at room temperature, and then counterstained, dehydrated with gradient ethanol, transparent with xylene, and sealed with neutral gum. Finally, the sections were observed under an optical microscope, and five fields were randomly selected to take pictures and analyzed with Image J.
Western blot
Tumor tissues of each group were taken, weighed, cut into homogenate, and distinct types of cells were lysed on ice by cell lysate containing protease inhibitor. The protein content was determined by the BCA kit. First, an equal amount of protein sample (20 mg) was extracted and denatured at 100 °C for 5 minutes. Then, the SDS-PAGE gel electrophoresis was performed, and the protein was transferred to the PVDF membrane. After blocking with 5% BSA at room temperature for 1–2 hours, the corresponding primary antibody was added and incubated overnight at 4 °C. The next day, horseradish peroxidase-labeled with secondary antibody was added after washing and was incubated at room temperature for one hour and washed. Finally, after adding the luminescent liquid, the gel imager was used for exposure and photographing, and the gray level value was calculated by Image J to calculate the relative expression level. β-actin serves as a reference for sample loading, and at least three independent experiments were performed.
Statistical analysis
SPSS17.0 software was used for statistical analysis. Normal distribution measurement data is expressed by, and a t-test was used for analysis. P<0.05 was considered statistically significant.
Results
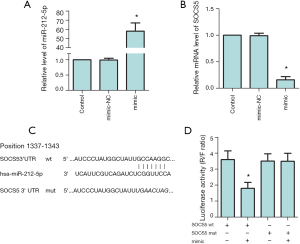
The targeting relationship between miR-212-5p and SOCS5
The RT-PCR detection results of miR-212-5p and SOCS5 mRNA levels are shown in Figure 1A,B (P<0.05). Compared with the control group, the expression of miR-212-5p in the mimic group was increased significantly (P<0.05), while the SOCS5 mRNA level was decreased significantly (P<0.05). Luciferase experiment was used to clarify the targeting relationship of miR-212-5p to SOCS5. The SOCS5 3’UTR sequence was obtained by searching the NCBI database. The luciferase activity was significantly reduced in HepG2 cells co-transfected with the wild-type SOCS5 3'-UTR and miR-212-5p (P<0.05, Figure 1C,D), and the results prove that miR-212-5p and SOCS5 have a direct targeting relationship.
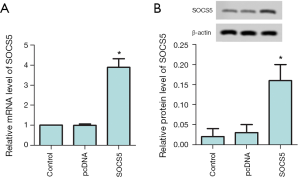
SOCS5 overexpression increases the mRNA and protein level of SOCS5
SOCS5 pcDNA vector was constructed, RT-PCR and Western blot were used to detect whether the overexpression is successful. The results are shown in Figure 2A,B. Compared with the control group, both mRNA and protein levels of SOCS5 in the pcDNA-SOCS5 group were significantly increased (P<0.05).
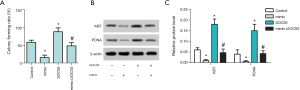
MiR-212-5p overexpression inhibits HepG2 cell proliferation and reduces Ki67 and PCNA protein levels
The results of cell proliferation in each group were detected by the cloning method, as shown in Figure 3A. Compared with the control group, the clonal formation rate of the mimic group was significantly reduced (P<0.05), while that of SOCS5 group was significantly increased (P<0.05). Also, the clone formation rate of the mimic+ SOCS5 group was significantly lower than that of SOCS5 group (P<0.05). The protein expression levels of Ki67 and PCNA in each group of cells detected by Western blot is shown in Figure 3B,C. Compared with the control group, Ki67 and PCNA protein levels in mimic group cells were significantly reduced (P<0.05), but Ki67 and PCNA protein levels in SOCS5 group cells were significantly increased (P<0.05). Moreover, Ki67 and PCNA protein levels in the mimic+ SOCS5 group were significantly lower than those in the SOCS5 group (P<0.05).
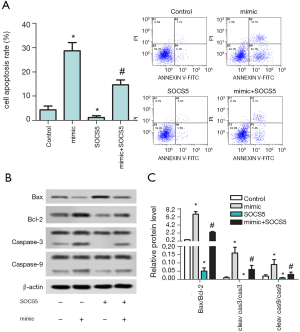
Overexpression of miR-212-5p promotes HepG2 cell apoptosis and increases the ratio of Bax/Bcl-2, cleared Caspase-3/Caspase-3, cleaved Caspase-9/Caspase-9
The results of flow cytometry detection of apoptosis in each group are shown in Figure 4A. Compared with the control group, the apoptosis rate in the mimic group increased significantly (P<0.05), but the apoptosis rate was decreased significantly in the SOCS5 group (P<0.05), and the apoptosis rate in mimic+ SOCS5 group was significantly higher than that in SOCS5 group (P<0.05). The protein levels of Bax, Bcl-2, Caspase-3, cleared caspase-3, Caspase-9, cleaved caspase-9 were detected by western blot in each group are shown in Figure 4B,C. Compared with the control group, the ratios of Bax/Bcl-2, cleared Caspase-3/Caspase-3, cleared Caspase-9/Caspase-9 in the mimic group were significantly increased (P<0.05), but those ratios were significantly decreased in SOCS5 group (P<0.05), and the ratios in mimic+ SOCS5 group were significantly higher than those in the SOCS5 group (P<0.05).
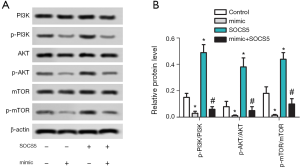
miR-212-5p overexpression reduces the ratio of p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR in HepG2 cells
The protein expression levels of PI3K, p-PI3K, AKT, p-AKT, mTOR, and p-mTOR in each group were detected by western blot and as shown in Figure 5A,B. Compared with the control group, the ratios of p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR in the mimic group were significantly decreased (P<0.05), but these ratios in SOCS5 group were significantly increased (P<0.05). Moreover, the ratios of p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR in the SOCS5 group were significantly lower than those in SOCS5 group (P<0.05).
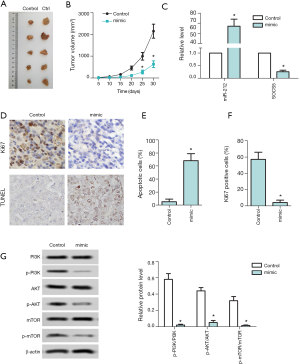
Effect of miR-212-5p overexpression on nude mice model with subcutaneous liver cancer transplantation
The tumor volume results after 30 days of detection are shown in Figure 6A,B. Compared with the control group, the tumor volume of the mimic group was significantly reduced on the 30th day (P<0.05). The expression levels of miR-212 and SOCS5 detected by RT-PCR are shown in Figure 6C. Compared with the control group, the miR-212 expression level of the mimic group was significantly increased (P<0.05), but the SOCS5 expression level was significantly reduced (P<0.05) The results of immunohistochemical detection of Ki67 positive cells are shown in Figure 6D,E,F. The number of Ki67 positive cells in the mimic group was significantly reduced (P<0.05) compared with the control group. The results of tumor cell apoptosis detected by TUNEL staining are shown in DE, and compared with the control group, the apoptosis rate in the mimic group increased significantly (P<0.05). The protein expression levels of PI3K, p-PI3K, AKT, p-AKT, mTOR, p-mTOR were detected by western blot and shown in Figure 6G. The ratio of PI3K/PI3K,p-AKT/AKT, and p-mTOR/mTOR decreased significantly in the mimic group (P<0.05) compared with the control group.
Discussion
Primary liver cancer is the fourth most common malignant tumor in China, and it poses a major threat to the lives and health of the Chinese people (16). Most patients with liver cancer are not suitable for radical surgery due to the presence of distant metastases at the time of initial diagnosis and the high recurrence rate of liver cancer (17). In addition to being a biomarker for early diagnosis of liver cancer, miRNA also acts as oncogenes and tumor suppressor genes in liver cancer and participates in the regulation of cell proliferation, apoptosis, invasion, and metastasis (18). Elucidating the molecular mechanisms involved in the development of hepatocellular carcinoma is essential to determine innovative therapeutic prognostic strategies. SOCS5 is expressed in various adult tissues, especially in primary B and T cells in the spleen, lymph nodes, thymus, and bone marrow (19). There is increasing evidence that SOCS5 is a tumor suppressor gene (20,21). This study found that miR-212-5p and SOCS5 have a direct targeting relationship, and miR-212-5p may be an effective potential target for the treatment of liver cancer.
HepG2 cells showed more similarity to human liver than the other cell lines in comparisons of the expression of cellular proteins (22). HepG2 cells are widely used as representative cells of liver cancer (23,24). Exosome extracted from HepG2 cells can induce human adipose-derived MSC to differentiate into cancer-associated myofibroblasts (25). Regulating the proliferation of tumor cells is an important way to treat tumors. Ki67 is a non-histone nucleoprotein present in the proliferating cell nucleus. It can recognize nuclear antigens in the cell cycle, and its expression level can show changes in cancer proliferation (26). Proliferating cell nuclear antigen (PCNA) is a nuclear protein related to the cell proliferation state. The higher the PNCA index, the faster the cell division and proliferation, which may promote the ability of the cell to obtain unlimited proliferation and eventually change the morphological structure and function of the cell (27). Ma and Li (28) found that the up-regulation of hsa-miR-212-5p expression can inhibit the proliferation and invasion of melanoma WM35 and A375 cells. This study found that the targeting inhibition of SOCS5 by miR-212-5p overexpression could reduce the protein expression levels of Ki67 and PCNA in HepG2 cells and decrease the Ki67 positive expression rate in nude mice. It is suggested that the targeting inhibition of SOCS5 by miR-212-5p overexpression could inhibit the proliferation of HepG2 cells and the growth of transplanted tumors in nude mice.
The essential strategy of tumor therapy is to induce apoptosis of tumor cells. Apoptosis is an important cellular mechanism that occurs in response to abnormalities and cell damage. Abnormal apoptosis reactions are quite common in the development of many types of human cancers (29). The anti-apoptotic proteins Bcl-2 and pro-apoptotic Bax proteins of the Bcl-2 family are key regulators of apoptosis. Increasing the ratio of Bax/Bcl-2 can promote apoptosis while reducing the Bax/Bcl-2 ratio will inhibit apoptosis, and in liver cancer cells, miRNA mainly affects apoptosis through the Bcl-2 family (30). The caspase family is the executor of apoptosis. When apoptosis-inducing factors such as Caspase-9 was activated, it can lead to a cascade of Caspase reactions, activating downstream apoptosis-inducing factors such as Caspase-3 (31). This study found that miR-212-5p overexpression targeting inhibition of SOCS5 could increase the ratio of Bax/Bcl-2, cleared Caspase-3/Caspase-3, cleared Caspase-9/Caspase-9 in HepG2 cells, promoting the apoptosis of tumor cells in nude mice. It is suggested that miR-212-5p overexpression can inhibit SOCS5 and promote HepG2 cell apoptosis.
The PI3K/Akt/mTOR signaling pathway is one of the three major signaling pathways in the cell, which can regulate the synthesis of various proteins, thereby widely participating in various regulation processes, such as cell proliferation, metabolism, survival, migration, and differentiation. Therefore, this signaling pathway has played an indispensable role in the occurrence and development of (32). The treatment of the PI3K/AKT/m TOR signaling pathway is one of the keys to anti-cancer therapy. Zhang et al. (33) found that stable knockdown of SOCS5 inhibited the invasion and metastasis of liver cancer cells through the autophagy pathway mediated by PI3K/Akt/mTOR. This study found that miR-212-5p overexpression targeting inhibition of SOCS5 has the effect of reducing the ratios of p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR in HepG2 cells, and nude mice xenografts. It is suggested that miR-212-5p overexpression targeting inhibition of SOCS5 regulates the malignant biological behavior of HepG2 cells and xenograft tumors through PI3K/Akt/mTOR signaling pathway.
In summary, miR-212-5p overexpression targeting inhibition of SOCS5 can inhibit the proliferation of HepG2 cells, inhibit the growth of transplanted tumors in nude mice, and promote apoptosis. These results may be achieved by activating the PI3K/Akt/mTOR signaling pathway. Whether miR-212-5p can be used as a marker for liver cancer diagnosis and treatment in the future, this experiment supplies a theoretical and experimental basis for it.
Acknowledgments
Funding: This study was supported in part by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2007
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2007
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2007). The authors report grants from Natural Science Foundation of Shaanxi Province, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The Committee for Animal Experiments of Second Affiliated Hospital of Air Force Medical University approved all of the experiments involving animals {No. 20181107[294]}.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Nenu I, Breaban I, Pascalau S, et al. The future is now: beyond first line systemic therapy in hepatocellular carcinoma. Transl Cancer Res 2019;8:S261-74. [Crossref]
- Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: Translating knowledge into practice. Clin Gastroenterol Hepatol 2015;13:2140-51. [Crossref] [PubMed]
- Hu C, Cui S, Zheng J, et al. MiR-875-5p inhibits hepatocellular carcinoma cell proliferation and migration by repressing astrocyte elevated gene-1 (AEG-1) expression. Transl Cancer Res 2018;7:158-69. [Crossref]
- Kabir TD, Ganda C, Brown RM, et al. A microRNA-7/growth arrest-specific 6/tyro3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma. Hepatology (Baltimore, Md) 2018;67:216-31. [Crossref] [PubMed]
- Xu CC, Wu LM, Sun W, et al. Effects of TGF-beta signaling blockade on human A549 lung adenocarcinoma cell lines. Mol Med Rep 2011;4:1007-15. [PubMed]
- Jia P, Wei G, Zhou C, et al. Upregulation of mir-212 inhibits migration and tumorigenicity and inactivates wnt/β-catenin signaling in human hepatocellular carcinoma. Technol Cancer Res Treat 2018;17:1533034618765221. [Crossref] [PubMed]
- Jia P, Wei G, Zhou C, et al. Upregulation of MiR-212 Inhibits Migration and Tumorigenicity and Inactivates Wnt/β-Catenin Signaling in Human Hepatocellular Carcinoma. Technol Cancer Res Treat 2018;17:1533034618765221. [Crossref] [PubMed]
- Li A, Wu J, Zhai A, et al. HBV triggers APOBEC2 expression through miR-122 regulation and affects the proliferation of liver cancer cells. Int J Oncol 2019;55:1137-48. [PubMed]
- Dou C, Wang Y, Li C, et al. MicroRNA-212 suppresses tumor growth of human hepatocellular carcinoma by targeting FOXA1. Oncotarget 2015;6:13216-28. [Crossref] [PubMed]
- Tiemin P, Fanzheng M, Peng X, et al. MUC13 promotes intrahepatic cholangiocarcinoma progression via EGFR/PI3K/AKT pathways. J Hepatol 2020;72:761-73. [Crossref] [PubMed]
- Sanchez-Mejias A, Kwon J, Chew XH, et al. A novel socs5/mir-18/mir-25 axis promotes tumorigenesis in liver cancer. Int J Cancer 2019;144:311-21. [Crossref] [PubMed]
- Zhang M, Liu S, Chua MS, et al. SOCS5 inhibition induces autophagy to impair metastasis in hepatocellular carcinoma cells via the PI3K/Akt/mTOR pathway. Cell Death Dis 2019;10:612. [Crossref] [PubMed]
- Sanchez-Mejias A, Kwon J, Chew XH, et al. A novel SOCS5/miR-18/miR-25 axis promotes tumorigenesis in liver cancer. Int J Cancer 2019;144:311-21. [Crossref] [PubMed]
- Yan D, Jin F, Lin Y. lncRNA HAND2-AS1 Inhibits Liver Cancer Cell Proliferation and Migration by Upregulating SOCS5 to Inactivate the JAK-STAT Pathway. Cancer Biother Radiopharm 2020;35:143-52. [Crossref] [PubMed]
- Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in china (2017 edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- Song T. Recent advances in surgical treatment of hepatocellular carcinoma. Drug Discov Ther 2015;9:319-30. [Crossref] [PubMed]
- Cheng CJ, Bahal R, Babar IA, et al. Microrna silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015;518:107-10. [Crossref] [PubMed]
- Sharma ND, Nickl CK, Kang H, et al. Epigenetic silencing of socs5 potentiates jak-stat signaling and progression of t-cell acute lymphoblastic leukemia. Cancer Science 2019;110:1931-46. [PubMed]
- Calvisi DF, Ladu S, Gorden A, et al. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest 2007;117:2713-22. [Crossref] [PubMed]
- Chen CY, Tsay W, Tang JL, et al. Socs1 methylation in patients with newly diagnosed acute myeloid leukemia. Genes Chromosomes Cancer 2003;37:300-5. [Crossref] [PubMed]
- Choi JM, Oh SJ, Lee SY, et al. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch Pharm Res 2015;38:691-704. [Crossref] [PubMed]
- Yang Y, Hou J, Shao M, et al. CXCL5 as an autocrine or paracrine cytokine is associated with proliferation and migration of hepatoblastoma HepG2 cells. Oncol Lett 2017;14:7977-85. [PubMed]
- Wang H, Yang M, Lin L, et al. HepG2 cells acquire stem cell-like characteristics after immune cell stimulation. Cell Oncol (Dordr) 2016;39:35-45. [Crossref] [PubMed]
- Luo F, Sun Z, Han Q, et al. Effect of Human Hepatocellular Carcinoma HepG2 Cell-derived Exosome on the Differentiation of Mesenchymal Stem Cells and Their Interaction. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017;39:312-7. [PubMed]
- Niikura N, Iwamoto T, Masuda S, et al. Immunohistochemical ki67 labeling index has similar proliferation predictive power to various gene signatures in breast cancer. Cancer Sci 2012;103:1508-12. [Crossref] [PubMed]
- Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of pcna–protein interactions for genome stability. Nat Rev Mol Cell Biol 2013;14:269-82. [Crossref] [PubMed]
- Ma L, Li J. MicroRNA-519d-3p inhibits cell proliferation and cell cycle G1/S transition in glioma by targeting CCND1. Biosci Biotechnol Biochem 2020;84:297-304. [Crossref] [PubMed]
- Labbé DP, Zadra G, Ebot EM, et al. Role of diet in prostate cancer: The epigenetic link. Oncogene 2015;34:4683-91. [Crossref] [PubMed]
- Zhang Y, Takahashi S, Tasaka A, et al. Involvement of microrna-224 in cell proliferation, migration, invasion, and anti-apoptosis in hepatocellular carcinoma. J Gastroenterol Hepatol 2013;28:565-75. [Crossref] [PubMed]
- Gao L, Mei S, Zhang S, et al. Cardio-renal Exosomes in Myocardial Infarction Serum Regulate Proangiogenic Paracrine Signaling in Adipose Mesenchymal Stem Cells. Theranostics 2020;10:1060-73. [Crossref] [PubMed]
- Djukom C, Porro LJ, Mrazek A, et al. Dual Inhibition of PI3K and mTOR Signaling Pathways Decreases Human Pancreatic Neuroendocrine Tumor (PNET) Metastatic Progression. Pancreas 2014;43:88-92. [Crossref] [PubMed]
- Zhang M, Liu S, Chua MS, et al. Socs5 inhibition induces autophagy to impair metastasis in hepatocellular carcinoma cells via the PI3K/AKT/MTOR pathway. Cell Death Dis 2019;10:612. [Crossref] [PubMed]


