
Plasma soluble human leukocyte antigen G predicts the long-term prognosis in patients with colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common tumor in the world, ranking fourth in the cause of cancer death (1). Although 60% to 70% of colorectal cancer patients are diagnosed with locally advanced tumors at the initial diagnosis, half of the patients will eventually relapse or metastasize after radical resection (2,3). Although the effectiveness of the current comprehensive treatment of colorectal cancer is significantly improved compared with before, and the five-year survival rate is also significantly improved. However, due to the large population with CRC, there are still many patients affected by poor prognosis. Therefore, finding suitable long-term prognostic indicators is essential for monitoring patients with a high risk of poor prognosis and, therefore, to improve prognosis. Many studies have shown that changes in the body’s immune status and inflammatory response are strictly related to the occurrence, development, and prognosis of tumors (4,5). Human leukocyte antigen G (HLA-G) belongs to non-classical HLA class I molecules, and is one of the immunosuppressive molecules for innate immunity and acquired immunity. The initial transcription product can be encoded to produce seven isomers, including four membrane-bound types (HLA-G1–HLA-G4) and three soluble types (HLA-G5–HLA-G7). Also, HLA -G1 can be hydrolyzed by matrix metalloproteinases to form another soluble HLA-G1 molecule (6). HLA-G molecules can bind to their corresponding receptors, including immunoglobulin-like transcript 2 (immunoglobulin-1ike transcript 2, ILT2), immunoglobulin-like transcript 4 (immunoglobulin-like transcript 4, ILT4), and Killer cell immunoglobulin-like receptor 2DL4 (KIR2DL4) participates in tumor immune escape by inhibiting the killing of natural killer (NK), inhibiting the proliferation of effector T cells and inducing the production of Treg cells and abnormally expressed HLA-G is often associated with various malignant tumors (7). This study aims to evaluate the long-term predictive value of soluble human leukocyte antigen G (soluble HLA-G, sHLA-G) in colorectal cancer through a two-center retrospective study since no research has investigate the long-term predictive value of this molecule in patients with CRC.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2211).
Methods
Study population
Patients who underwent radical colorectal cancer surgery in two hospitals from April 2010 to March 2015 were enrolled according to the inclusion criteria: (I) pathologically confirmed colon or rectal cancer during or after surgery; (II) surgery and other anti-tumor treatment for the first time; (III) acquisition of frozen plasma before surgery; (IV) age ≥18 years old; (V) completed more than five years of follow-up or died within five-year follow-up; exclusion criteria: (I) connective tissue disease; (II) severe heart failure; (III) severe renal function decline; (IV) active liver disease. All procedures performed in this study were in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital and the Ethics Committee of Shaanxi Fourth People’s Hospital. The ethics committee waived informed consent.
Data collection
The Patient’s baseline data, including demographic data, preoperative blood test indicators, test results, surgical and pathological results, and five-year follow-up results, were collected. The patients’ preoperative plasma sHLA-G baseline level was detected. According to whether they survived at five years of follow-up, they were divided into two groups. Univariate and multivariate analysis is used to factors associated with the rate of five-year survival. The ROC curve was generated to correlate sHLA-G with the rate of five-year survival, and the cutoff value of sHLA-G for predicting five-year survival was obtained. According to this cutoff value, patients were divided into high-level and low-level sHLA-G groups, and baseline data of the two groups were compared. The Kaplan-Meier survival curve was used to analyze the difference of long-term prognosis between the two groups.
Samples test
For each patient before surgery, 10 mL of blood was acquired, centrifuged at 1,800 ×g for 10 minutes at room temperature to separate plasma, and stored frozen at −80 °C until use. The concentration of plasma sHLA-G was detected with a specific enzyme linked immunosorbent assay (ELISA) kit (BioVendor Laboratory Medicine, Inc., Brno, Czech Republic), and operated according to the manual instructions. The detection limit of this kit was 0.6 U/mL. Other baseline test results include white blood cell count, lymphocyte count, neutrophil count, red blood cell count, hemoglobin concentration, platelet count, urine routine, fecal occult blood, liver function, kidney function, electrolytes, carcinoembryonic antigen (CEA) before the operation was collected and repeated at follow-up.
Follow-up
All patients were followed up at three months, six months, and one year after surgery and then followed up once a year. Follow-up contents included: laboratory test described as before, liver ultrasound, colonoscopy. Abdominal computed tomography (CT) was repeated for patients with lymph node metastasis detected in the preoperative examination or during operation. Other information, including survival or not, second-time surgery was obtained.
Statistical analysis
Statistical processing was performed using SPSS17.0 statistical software. Data that conformed to the normal distribution were expressed as mean ± standard deviation, and comparison between groups was conducted using the student test; those that did not conform to the normal distribution were expressed as median, and comparison between groups was conducted using the rank-sum test. The qualitative data are expressed in numerical values and percentages, and comparisons between groups are performed using the X2 test or Fisher’s exact test. The ROC curve was used to correlate sHLA-G with the rate of five-year survival. Kaplan-Meier survival curve was used to compare the prognosis between groups with the different plasma levels of sHLA-G. P<0.05 was considered statistically significant.
Results
Baseline characteristics and follow-up outcome
According to the exclusion criteria, 1,037 patients with colorectal cancer were included in the final analysis (Table 1). The patients in this study were 34 years old to 72 years old, with an average of 62.3±12.7 years old, including 592 elderly patients (≥60 years old) and 445 young and middle-age patients (<60 years old). Almost all this population was male (58.1%), and over 50% of these patients were diagnosed with rectal cancer (56.1%). As for Tumor Node Metastasis (TNM) stages, 32.5% of patients were at stage II, 50% were at stage III. The diameter of the tumor in 1/3 patients was less than 4 cm. See Table 1 for details. The majority (71.7%) of patients received adjuvant chemoradiation, but the proportion of elderly patients receiving adjuvant chemoradiation was lower than non-elderly patients (386/592 vs. 358/445, X2=29.132, P<0.001).
Table 1
| Items | Results |
|---|---|
| Age (years) | 62.3±12.7 |
| Sex (n, %) | |
| Male | 603 (58.1) |
| Female | 434 (41.9) |
| Rectal (n, %) | 582 (56.1) |
| Colon (n, %) | 455 (43.9) |
| Diameter of tumor (n, %) | |
| ≥4 cm | 413 (39.8) |
| <4 cm | 624 (60.2) |
| Differentiation (n, %) | |
| Low | 282 (27.2) |
| High-moderate | 755 (72.8) |
| T stage (n, %) | |
| 1–2 | 475(45.8) |
| 3–4 | 562 (54.2) |
| N stage (n, %) | |
| 0 | 487 (47.0) |
| 1–2 | 550 (53.0) |
| M stage (n, %) | |
| 0 | 993 (95.8) |
| 1 | 44 (4.2) |
| Tumor stage (n, %) | |
| I | 144 (13.9) |
| II | 337 (32.5) |
| III | 519 (50.0) |
| IV | 37 (3.6) |
| WBC (×109/mL) | 5.8±2.4 |
| NEU (×109/mL) | 3.5±1.4 |
| Hb (g/L) | 129.6±17.2 |
| PLT (×109/mL) | 163.5±48.1 |
| ALT (U/L) | 31.2±13.6 |
| Cr (μmol/L) | 71.9±16.4 |
| TC (mmol/L) | 5.7±1.5 |
| TG (mmol/L) | 1.9±1.1 |
| CEA (ng/mL) | 7.2±2.7 |
| Hs-CRP (mg/L) | 4.9±2.5 |
| Smoke (n, %) | 297 (28.6) |
| Alcohol (n, %) | 411 (39.6) |
| FH (n, %) | 137 (13.2) |
| R&C (n, %) | 744 (71.7) |
| sHLA-G (U/L) | 48.2±13.8 |
WBC, white blood cell; NEU, neutrophils; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; Cr, creatine; TC, total cholesterol; TG, triglyceride; CEA, cancer embryo antigen; hs-CRP, high sensitivity C reaction protein; FH, family history; R&C, radiotherapy or chemotherapy; sHLA-G, soluble human leukocyte antigen G.
Univariate and multivariate analysis of five-year survival of colorectal cancer patients
During the five-years follow-up, 302 patients (29.1%) died, 735 patients (70.9%) survived, and 73 patients (7.0%) were diagnosed colorectal cancer again. Patients were divided into two groups: patients who survived (survival group) and patients who died (death group) during follow-up. The baseline data of the two groups of patients are compared (Table 2). After the initial univariate analysis, a multivariate analysis was performed (Table 3). The age of the patients in the survival group was significantly lower than the death group, and the proportion of male patients was higher in the survival group. The proportion of patients at TNM stage I/II in the survival group was significantly higher than that in the death group. The patients in the death group had a higher proportion of first-degree relatives of their family history of colorectal cancer, and their plasma sHLA-G level was significantly higher than those in the survival group, and their blood CEA level was significantly higher than those in the survival group. From these results, we select parameters with a P value not greater than 0.1 for further univariate and multivariate analysis. The results are shown in Table 3. It can be seen that age, tumor differentiation, family history, TNM stage, and preoperative plasma sHLA-G levels are predictive factors for the five-year prognosis of colorectal cancer patients.
Table 2
| Items | Group of survival (n=735) | Group of death (n=302) | t/X2 value | P value |
|---|---|---|---|---|
| Age (years) | 60.1±13.6 | 67.7±14.8 | −7.965 | <0.001 |
| Male (n, %) | 447 (60.8) | 156 (51.7) | 7.381 | 0.007 |
| Rectal (n, %) | 402 (54.7) | 180 (59.6) | 2.095 | 0.148 |
| Colon (n, %) | 333 (45.3) | 122 (40.4) | ||
| Diameter of tumor (n, %) | 0.209 | 0.647 | ||
| ≥4 cm | 296 (40.3) | 117 (38.7) | ||
| <4 cm | 439 (59.7) | 185 (61.3) | ||
| T stage (n, %) | 2.009 | 0.1556 | ||
| 1–2 | 347 (47.2) | 128 (42.4) | ||
| 3–4 | 388 (52.8) | 174 (57.6) | ||
| N stage (n, %) | 59.185 | <0.001 | ||
| 0 | 289 (39.3) | 198 (65.6) | ||
| 1–2 | 446 (60.7) | 104 (34.4) | ||
| M stage (n, %) | ||||
| 0 | 706 (96.1) | 287 (95.0) | 0.550 | 0.489 |
| 1 | 29 (3.9) | 15 (5.0) | ||
| Tumor stage (n, %) | 39.887 | <0.001 | ||
| I/II | 387 (52.7) | 94 (31.1) | ||
| III/IV | 348 47.3) | 208 (68.9) | ||
| WBC (109/mL) | 5.9±2.5 | 5.6±2.3 | 1.796 | 0.073 |
| NEU (109/mL) | 3.5±1.4 | 3.5±1.4 | 0.000 | 1.000 |
| Hb (g/L) | 130.2±17.8 | 128.1±18.3 | 1.712 | 0.087 |
| PLT (109/mL) | 167.2±52.5 | 154.5±55.1 | 1.236 | 0.217 |
| ALT (U/L) | 30.4±11.9 | 33.1±12.6 | -1.208 | 0.227 |
| Cr (μmol/L) | 71.3±15.3 | 73.6±16.1 | -1.883 | 0.060 |
| TC (mmol/L) | 5.7±1.6 | 5.7±1.8 | 0.000 | 1.000 |
| TG (mmol/L) | 1.9±0.9 | 1.9±1.2 | 0.000 | 1.000 |
| CEA (ng/mL) | 7.1±2.5 | 7.4±2.9 | -1.674 | 0.095 |
| Hs-CRP (ng/L) | 5.0±2.4 | 4.7±2.6 | 1.784 | 0.075 |
| Smoke (n, %) | 219 (29.8) | 78 (25.8) | 1.649 | 0.199 |
| Alcohol (n, %) | 281 (38.2) | 130 (43.0) | 2.074 | 0.150 |
| FH (n, %) | 74 (10.1) | 63 (20.9) | 21.746 | <0.001 |
| R&C (n, %) | 533 (72.5) | 211 (69.9) | 0.741 | 0.389 |
| sHLA-G (U/L) | 41.4±16.9 | 64.7±23.2 | -17.989 | <0.001 |
WBC, white blood cell; NEU, neutrophils; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; Cr, creatine; TC, total cholesterol; TG, triglyceride; CEA, cancer embryo antigen; hs-CRP, high sensitivity C reaction protein; FH, family history; R&C, radiotherapy or chemotherapy; sHLA-G, soluble human leukocyte antigen G.
Table 3
| Items | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.572 (1.108–1.836) | 0.008 | 1.602 (1.115–1.918) | 0.007 | |
| Male | 1.114 (0.947–1.427) | 0.205 | 1.200 (0.985–1.513) | 0.186 | |
| Differentiation | 1.402 (1.074–1.739) | 0.017 | 1.483 (1.104–1.805) | 0.021 | |
| FH | 1.684 (1.121–1.968) | 0.028 | 1.539 (1.117–1.933) | 0.031 | |
| TNM stage | 2.015 (1.328–2.415) | 0.006 | 2.022 (1.441–2.571) | 0.009 | |
| sHLA-G | 1.906 (1.377–2.305) | 0.010 | 1.784 (1.319–2.283) | 0.012 | |
FH, family history; sHLA-G, soluble human leukocyte antigen G.
The cutoff point of soluble human leukocyte antigen G
A receiver operating characteristic (ROC) curve was used to evaluate the ability of the plasm level of sHLA-G discriminated between patients who survived and patients who did not survive during the five-year follow-up (Figure 1). The area under the ROC curve was 0.766, and the best cutoff value of sHLA-G for predicting the five-year survival of colorectal cancer patients was 50.8 U/mL. The specificity at this point was 78.1%, and the sensitivity was 72.3%. According to the cut-off value, the patients were divided into high-level group (sHLA-G ≥50.8 U/mL) and low-level group (sHLA-G <50.8 U/mL). A comparison of baseline data between these two groups (Table 4) shows that preoperative sHLA-G has a strong relationship with patients’ conditions. Low-level patients have smaller tumors, a higher proportion of stage I and stage II, and a lower proportion of positive family history CRC.
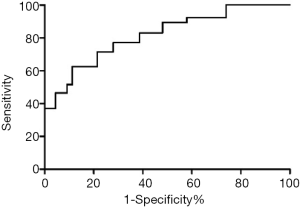
Table 4
| Items | High level (n=415) | Low level (n=622) | t/X2 value | P value |
|---|---|---|---|---|
| Age (years) | 63.1±13.6 | 61.8±12.8 | 1.563 | 0.119 |
| Male (n, %) | 219 (52.8) | 384 (61.7) | 8.221 | 0.004 |
| Rectal (n, %) | 239 (57.6) | 343 (55.1) | 0.605 | 0.437 |
| Colon (n, %) | 176 (42.4) | 279 (44.9) | ||
| Diameter of tumor (n, %) | 11.969 | 0.001 | ||
| ≥4 cm | 192 (46.3) | 221 (35.5) | ||
| <4 cm | 223 (53.7) | 401 (64.5) | ||
| T stage (n, %) | 13.696 | <0.001 | ||
| 1–2 | 161 (38.8) | 314 (50.5) | ||
| 3–4 | 254 (61.2) | 308 (49.5) | ||
| N stage (n, %) | 8.454 | 0.004 | ||
| 0 | 172 (41.4) | 315 (50.6) | ||
| 1–2 | 243 (58.6) | 307 (49.4) | ||
| M stage (n, %) | 0.037 | 0.848 | ||
| 0 | 398 (95.9) | 595 (95.7) | ||
| 1 | 17 (4.1) | 27 (4.3) | ||
| Tumor stage (n, %) | 44.601 | <0.001 | ||
| I | 39 (9.4) | 105 (16.9) | ||
| II | 108 (26.0) | 229 (36.8) | ||
| III | 241 (58.1) | 278 (44.7) | ||
| IV | 27 (6.5) | 10 (1.6) | ||
| WBC (109/mL) | 5.7±2.2 | 5.9±2.4 | −1.359 | 0.175 |
| NEU (109/mL) | 3.4±1.5 | 3.6±1.6 | −2.022 | 0.044 |
| Hb (g/L) | 128.5±18.9 | 130.3±19.6 | −1.470 | 0.142 |
| PLT (109/mL) | 164.4±50.2 | 162.9±49.3 | 0.477 | 0.634 |
| ALT (U/L) | 32.7±14.1 | 30.2±13.9 | 2.708 | 0.007 |
| Cr (μmol/L) | 73.5±18.8 | 70.8±17.4 | 2.370 | 0.018 |
| TC (mmol/L) | 5.4±1.6 | 5.9±1.9 | −4.417 | <0.001 |
| TG (mmol/L) | 1.8±0.8 | 2.0±1.3 | −2.800 | 0.005 |
| CEA (U/L) | 7.0±2.0 | 7.3±1.9 | −2.439 | 0.015 |
| Hs-CRP (ng/L) | 5.1±2.8 | 4.8±2.6 | 1.765 | 0.078 |
| Smoke (n, %) | 129 (31.1) | 168 (27.0) | 2.022 | 0.155 |
| Alcohol (n, %) | 175 (42.2) | 236 (37.9) | 1.859 | 0.173 |
| FH (n, %) | 73 (17.6) | 64 (10.3) | 11.572 | 0.001 |
| R&C (n, %) | 262 (63.1) | 482 (77.5) | 25.319 | <0.001 |
| sHLA-G (U/L) | 56.8±15.5 | 42.5±14.7 | 15.016 | <0.001 |
sHLA-G, soluble human leukocyte antigen G; WBC, white blood cell; NEU, neutrophils; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; Cr, creatine; TC, total cholesterol; TG, triglyceride; CEA, cancer embryo antigen; hs-CRP, high sensitivity C reaction protein; FH, family history; R&C, radiotherapy or chemotherapy.
Comparison of five-year follow-up outcomes of patients with different levels of sHLA-G
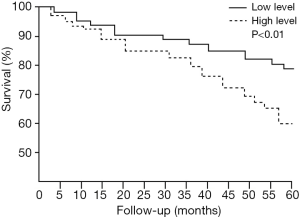
The comparison of the follow-up outcomes between the two groups found the proportion of disease-free survival, recurrence, and death of patients in the low-level group during five-year follow-up was significantly lower than those in the high-level group. Furthermore, the success rate of second surgery in the low-level group was higher than that in the high-level group (28/35 vs. 22/38), see Table 5 for details. The Kaplan-Meier survival curve showed that the prognosis of patients in the low-level group was significantly better than that in the high-level group (Figure 2).
Table 5
| Events | High-level group (n=415) | Low-level group (n=622) | t/X2 value | P value |
|---|---|---|---|---|
| Disease-free survival (n, %) | 229 (55.2) | 433 (69.6) | 22.463 | <0.001 |
| Death (n, %) | 167 (40.2) | 135 (21.7) | 41.437 | <0.001 |
| Recurrence (n, %) | 38 (9.2) | 35 (5.6) | 4.739 | 0.030 |
| Second surgery (n, %) | 22 (57.9) | 28 (80.0) | 4.125 | 0.042 |
Discussion
In this study, we retrospectively analyzed the data of 1,037 patients who underwent radical colorectal cancer resection in the two hospitals for five years and found the baseline plasma sHLA-G level was strongly associated with the five-year survival after surgery. We found that age, differentiation, family history, TNM stage, and preoperative baseline level of sHLA-G are essential factors related to the survival of colorectal cancer patients. In this study, the optimal cutoff value for sHLA-G to predict five-year survival after surgery was 50.8 U/mL, with a specificity of 78.1% and a sensitivity of 72.3%. According to this point, the patients were divided into a low-level group and a high-level group, which showed the differentiation level, TNM stage, and five-year survival rate of the low-level group were better than those of the high-level group. The Kaplan-Meier survival curve showed that the five-year prognosis of patients in the low-level group was better than that in the high-level group.
The incidence and death of CRC in China have increased in recent years. 2015 Chinese cancer statistics showed that the incidence and mortality of colorectal cancer in China ranks fifth among all malignant tumors, including 376,000 new cases and 191,000 deaths. The incidence of CRC in urban areas is much higher than that in rural areas, and most patients are already in the middle and late stages when they are diagnosed (8). However, with the wide use of colonoscopy, the improvement of surgical methods, and the progress of postoperative adjuvant radiotherapy and chemotherapy, the current treatment effectiveness of colorectal cancer and its prognosis have been significantly improved compared with the previous situation. However, due to the large population in China, the number of patients with colorectal cancer is enormous. Although more patients undergo radical surgery for colorectal cancer, many patients still have a poor prognosis. Therefore, how to predict the patient’s prognosis more accurately is crucial to monitor high-risk patients and help discover adverse events and provide effective intervention early. Previous studies have found some useful predictors. First of all, some traditional predictors, including the tumor differentiation, TNM stage, and the age of the patient, are strongly associated with patients’ prognosis. Other parameters including changes in several neutrophils in blood indicators (9), neutrophil/lymphocyte ratio (10), plasma fibrinogen (11), serum thymidine kinase 1 (12), peripheral blood lymphocytes, and mononuclear cell ratio (13), microRNA (14), etc., also have certain predictive values for the postoperative prognosis of colorectal cancer patients. However, these predictors are not closely related to the mechanism of tumorigenesis and development and are easily disturbed by many factors, including neutrophils, which are easily disturbed by the infection status of patients. Therefore, there are still many studies to explore more suitable blood indicators for the long-term prognosis of colorectal cancer. HLA-G is one of the most critical immunosuppressive molecules in the human body. It is only expressed at the maternal-fetal interface, and some immune exempted tissues under physiological conditions (15), while abnormally expressed HLA-G is often associated with various malignant tumors, viral infections, and inflammations (16-18). Earlier studies have shown that 70.7% of colorectal cancer tissues express HLA-G, and the expression status of HLA-G is closely related to the prognosis of patients.
Further studies have found that plasma sHLA-G can be used as colorectal cancer independent prognostic factors for patients (19,20). Also, Zhu et al. found that serum sHLA-G levels can be used for differential diagnosis of benign and malignant colorectal diseases, suggesting that HLA-G can be used as a biomarker for the diagnosis and prognosis of colorectal cancer (21). However, there are several problems with these studies. Some studies have a smaller sample size, some studies only aim to assess the severity of the disease, and some studies have relatively shorter follow-up duration. At present, there are consensus and guidelines regarding the screening and early diagnosis of colorectal cancer (22,23). For the long-term prognosis of patients with colorectal cancer after radical surgery, the study is still inadequate. Simultaneously, due to the wide use of colonoscopy and the gradual increase in people’s awareness of physical examination, the diagnostic rate of colorectal cancer of early and middle stages has dramatically increased, resulting in the current demographic characteristics of colorectal cancer patients differing from the those of the past (1-3), which also affects the value of traditional risk factors or predictors. Therefore, it is necessary to evaluate further the existing predictors for the new research results.
This study confirmed that sHLA-G could be used as a long-term prognostic indicator for patients with colorectal cancer after radical surgery. These findings are consistent with the results of other studies (IJK). Simultaneously, however, the sample size involved in this study is larger, and the follow-up time is longer. The ability of sHLA-G levels to predict prognosis during follow-up has already been revealed at the end of 3 years follow-up after surgery (survival rate 76.2% vs. 85.1%, P=0.032). From this result, we can select patients at elevated risk of poor prognosis to develop a personalized follow-up plan.
Moreover, our research results showed that the preoperative baseline level of sHLA-G is not only related to five-year survival, but also has a statistically significant correlation with disease-free survival, tumor recurrence, and re-operation. Therefore, for colorectal cancer, patients with a high baseline level of sHLA-G, regular colonoscopy, and other examinations are of clinical significance. Limitations of this study: Due to the smaller sample size, this study cannot combine sHLA-G with other predictive indicators to establish a predictive score, and its predictive validity may be relatively weak. Concurrently, because most colorectal cancer patients are middle-aged, and older adults and the middle-aged and older adults often have other diseases and receive certain drug treatment. This study did not analyze the effect of drugs on the circulating level of sHLA-G. Future research should focus on these points.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2211
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2211
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2211). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital and the Ethics Committee of Shaanxi Fourth People’s Hospital. The ethics committee waived informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85. [Crossref] [PubMed]
- Henry JT, Johnson B. Current and evolving biomarkers for precision oncology in the management of metastatic colorectal cancer. Chin Clin Oncol 2019;8:49. [Crossref] [PubMed]
- Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019;7:609. [Crossref] [PubMed]
- Clark SK. Management of genetically determined colorectal cancer. Surgeon 2019;17:165-71. [Crossref] [PubMed]
- Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361-75. [Crossref] [PubMed]
- Cai Z, Wang L, Han Y, et al. Immunoglobulin like transcript 4 and human leukocyte antigen G interaction promotes the progression of human colorectal cancer. Int J Oncol 2019;54:1943-54. [PubMed]
- Li X, Sheng Z, Sun Y, et al. Human leukocyte antigen-G upregulates immunoglobulin-like transcripts and corrects dysfunction of immune cells in immune thrombocytopenia. Haematologica 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Kang ZC. Correlation between neutrophils changes and prognosis of colorectal cancer. Chin J Gen Surg 2018;33:845-8.
- Ashizawa N, Furuya S, Katsutoshi S, et al. Clinical Significance of Dynamic Neutrophil-lymphocyte Ratio Changes in Patients With Colorectal Cancer. Anticancer Res 2020;40:2311-7. [Crossref] [PubMed]
- Li M, Wu Y, Zhang J, et al. Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16974. [Crossref] [PubMed]
- Dang L, Ma H, Hei A, et al. A meta-analysis of serological thymidine kinase 1 as a marker for colorectal benign and malignant tumor risk assessment. Mol Clin Oncol 2020;12:440-50. [PubMed]
- Ichikawa N, Homma S, Yoshida T, et al. An increase in the peripheral lymphocyte-to-monocyte ratio after primary site resection is associated with a prolonged survival in unresectable colorectal carcinoma. Surg Today 2020;50:604-14. [Crossref] [PubMed]
- Gmerek L, Martyniak K, Horbacka K, et al. MicroRNA regulation in colorectal cancer tissue and serum. PLoS One 2019;14:e0222013. [Crossref] [PubMed]
- Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, et al. HLA-G: An Immune Checkpoint Molecule. Adv Immunol 2015;127:33-144. [Crossref] [PubMed]
- Wan R, Wang ZW, Li H, et al. Human Leukocyte Antigen-G Inhibits the Anti-Tumor Effect of Natural Killer Cells via Immunoglobulin-Like Transcript 2 in Gastric Cancer. Cell Physiol Biochem 2017;44:1828-41. [Crossref] [PubMed]
- Amiot L, Vu N, Samson M. Biology of the immunomodulatory molecule HLA-G in human liver diseases. J Hepatol 2015;62:1430-7. [Crossref] [PubMed]
- Gomes RG, Brito CAA, Martinelli VF, et al. HLA-G is expressed in intestinal samples of ulcerative colitis and Crohn's disease patients and HLA-G5 expression is differentially correlated with TNF and IL-10 cytokine expression. Hum Immunol 2018;79:477-84. [Crossref] [PubMed]
- Zhang RL, Zhang X, Dong SS, et al. Predictive value of different proportion of lesion HLA-G expression in colorectal cancer. Oncotarget 2017;8:107441-51. [Crossref] [PubMed]
- Li JB, Ruan YY, Hu B, et al. Importance of the plasma soluble HLA-G levels for prognostic stratification with traditional prognosticators in colorectal cancer. Oncotarget 2017;8:48854-62. [Crossref] [PubMed]
- Zhu CB, Wang CX, Zhang X, et al. Serum sHLA-G levels: a useful indicator in distinguishing colorectal cancer from benign colorectal diseases. Int J Cancer 2011;128:617-22. [Crossref] [PubMed]
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250-81. [Crossref] [PubMed]
- Li ZS, Jin ZD, Linghu EQ, et al. Expert consensus on the screening process of early colorectal cancer in China. Natl Med J China 2019;99:2961-70.
(English Language Editor: J. Chapnick)


