
Clinicopathological characteristics and gene analysis of pulmonary granular cell tumor in three cases and a systematic review
Introduction
Granular cell tumor (GCT) is a rare tumor characterized by abundant intracytoplasmic granules, which is benign in most cases and can arise in multiple anatomical sites. It occurs most commonly in the skin, tongue and breast, while it is rarely found in the lungs (1). Previous studies have suggested that it most likely originates from Schwann cells (2,3). A recent study has demonstrated that inactivating mutations of ATP6AP1 and ATP6AP2 may be the oncogenic drivers of GCT (4). Although these studies have explored the pathogenesis of GCT, the mechanism and genetic landscape of GCT still remain unclear.
Pulmonary GCT (PGCT) accounts for 6–10% of all GCTs, among which a handful of malignant PGCT have been reported (5). Due to the rarity of PGCT, there is a scarcity of data on this tumor, while most of the existing studies have focused on case report or retrospective analysis of small samples. Therefore, systematic review with large samples on PGCT is needed, especially on malignant PGCT.
The purpose of this study was to assess the clinicopathological characteristics and gene analysis of the tumor cases with a systematic review of literature. Therefore, we analyzed the clinical presentation, radiological image, pathological feature, treatment options and follow-up in 3 patients diagnosed with PGCT at Shanghai Chest Hospital. We furthermore systematically reviewed a total of 81 cases of PGCT, in which 78 cases were reported across 43 different studies.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-133).
Methods
Information sources and search strategy
We searched the Shanghai Chest Hospital database for records between January 2000 and July 2019 and found a total of 3 cases of PGCT (ranging from June 2015 to June 2019), summarized the clinical data of these three patients and conducted follow-up. The last follow-up date was July 8, 2019.
We searched articles in PubMed, EMBASE and Web of Science from 1999 to 2019 using the following search keywords: GCT, lung, pulmonary, trachea, bronchial, bronchus, endobronchial, tracheobronchial. Reference lists from the included studies were also searched, with the last search being performed on July 8, 2019.
The inclusion criteria were as follows: (I) all selected PGCT patients should be pathologically diagnosed; (II) primary PGCT; (III) patients had relevant information on examination and treatment. The exclusion criteria were: (I) the subjects were non-human; (II) GCTs of other organs; (III) non-primary PGCT; (IV) studies published before 1999; (V) repeated cases; (VI) studies that were not published in English language. The study selection process was done independently by two authors and any discrepancies were resolved through discussion with the third author. Flow diagram of study selection is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received the approval of the Ethics Committee in Shanghai Chest Hospital (Ethics number: KS2014) and all patients signed an informed consent.
Data collection
Two authors independently collected the following data from the included studies, including but not limited to: age, gender, clinical symptoms, tumor location, radiological images, pathological features, laboratory results, therapy, and outcomes.
Criteria for malignant PGCT
In our study, the histologic malignancy of PGCT was identified using the criteria proposed by Fanburg-Smith et al. (6). The criteria were as follows: (I) tumor necrosis; (II) spindle cells; (III) increased mitotic activity (>2/10 high power field); (IV) vesicular nuclei with prominent nucleoli; (V) pleomorphism (cellular and/or nuclear); (VI) high nucleocytoplasmic ratio. Patients who met three or more of these criteria were considered to have pathologically malignant disease.
Gene sequencing of the tissue
According to the manufacturer’s instructions, the tissue DNA of two cases was extracted from paraffin-embedded sections by a QIAamp DNA FFPE tissue kit (Qiagen, Carlsbad, CA, USA), and the DNA concentration was measured using Qubit dsDNA assay (Thermo Fisher Scientific, Waltham, MA, USA).
DNA fragmentation was performed using the Covaris M220 Focused-ultrasonicator (Woburn, MA, USA) and then end repair, phosphorylation and adaptor ligation. The sections from 200 to 400 bp were selected using the AMPure beads (Agencourt AMPure XP Kit, Beckman Coulter, CA, USA) and then hybridized with the capture probe bait and selected by magnetic bead hybridization and PCR amplification. Subsequently, highly sensitive DNA assays were performed to assess the quality and size of all fragments. The OncoScreen panel (Burning Rock Biotech Ltd., Guangzhou, China) was used to assess genetic profile of all tissue samples by capture-based targeting depth sequencing method, covering 202 MB of the human genome region, including all 520 exons and key introns (see the table at http://fp.amegroups.cn/cms/def8760bf8f5c47d35cf85cb63897030/TCR-20-133-1.pdf). The quality and size of DNA were assessed by high sensitivity DNA assays using a biological analyzer. All indexed samples were sequenced on the NextSeq 500 (Illumina, Inc., Madison, WI, USA) with pair-end reads.
Statistical analysis
Data were collected and summarized in Microsoft Excel 16.0 (Microsoft Inc., Redmond, Washington, USA).
Results
Three cases in Shanghai Chest Hospital
Case 1
A 56-year-old male with a history of 100-pack-year cigarette smoking was admitted to our hospital due to an abnormal shadow on chest CT scan for evaluation of one-month cough at the local hospital requested. Physical examination on admission was within normal limits. Routine laboratory results were negative. Bronchoscopy at local hospital showed a neoplasm obstructing the orifice of the right upper lobe bronchus. The bronchoscopic biopsy showed the presence of spindle cells containing eosinophilic granules, which were positive for S-100 staining. This indicated a probable PGCT. The chest CT was subsequently re-performed revealing a soft tissue mass in the right upper lobe bronchus, which could be enhanced through the repeat scanning (Figure 2A,B). A right upper sleeve lobectomy under single-hole thoracoscopy was performed. Macroscopically, a 1.2 cm × 1.0 cm × 1.0 cm ill-defined intraluminal mass on the right upper bronchial orifice was located at 0.2 cm from the upper edge of the lesion and 0.8 cm from the lower edge of the lesion. Microscopically, there were no areas of necrosis or signs of mitoses. The surgical margins and three lymph nodes in the peri-bronchial region were tumor free. Immunohistochemically, strong and diffuse nuclear and cytoplasmic reactivity for S-100 protein and CD56 in the neoplastic cells. Stains for SYN and TFE3 were weak positive. Stains for CK, CgA, NSE, ACTIN, DES, NSE, HMB-45, and EMA were negative. The proliferative index with Ki-67 was 1% in the tumor cells. Based on the described features, benign GCT was diagnosed. The patient was discharged 6 days after surgery, and showed no tumor recurrence or metastatic disease after 2 years.

Case 2
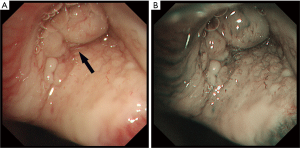
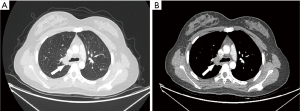
A 16-year-old girl presented at the emergency department of a local hospital complaining of productive cough with yellow or green purulent sputum, fever, and right chest pain for 7 days. Blood routine examination showed white blood cells 21.1×109/L, neutrophils percentage 82.1%, C-reactive protein 115.85 mg/L. A chest CT scan showed segmental atelectasis of the right upper lobe. She was treated with intravenous antibiotics for one week. The re-examination CT showed an increase in the size of atelectasis with the progressed right upper lobe. Then the patient was referred to our hospital. Physical examination was unremarkable except for decreased breath sounds over the right upper lung field. The blood routine examination and the serum levels of tumor markers were within normal limits. Bronchoscopy was performed revealing a smooth, lobulated, polypoid, whitish mass adherent to the bronchial wall and completely obstructing the orifice of the right upper lobe bronchus (Figure 3). The bronchoscopic biopsy demonstrated sheets of closely packed round cells in the bronchial mucosa, with abundant eosinophilic cytoplasm and mild nuclear atypia, which indicated a possible diagnosis of PGCT. The contrast-enhanced chest CT scan revealed irregular stenosis in the right main bronchus (Figure 4A,B). A solitary round-like substantial mass with lobulated and spiculated signs was observed in the right hilar region measuring approximately 25 mm in diameter. Given the extent of her disease, a right upper sleeve lobectomy and lymph node dissection were performed under video-assisted thoracic surgery (VATS). Grossly, the tumor was a solitary mass, measuring 2.5 cm × 2.0 cm × 1.5 cm in size. Microscopically, the well-circumscribed mass infiltrated all layers of the bronchial walls and the surrounding nerves. No obvious necrosis or mitotic figures were seen in the sections. The surgical resection margins and all regional lymph nodes were tumor-free. The immunohistochemical study demonstrated the following (Figure 5): The tumor cells were positive for S-100, CD68, SOX10, VIM and NSE, and negative for CgA, ACTIN, CK, HMB-45. The Ki-67 proliferative index was 5%. The cytoplasmic granules were PAS-negative. A benign PGCT was diagnosed. The patient was discharged 8 days after surgery. She was asymptomatic after 3 months.
Case 3
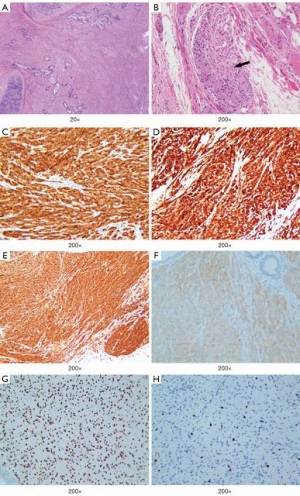
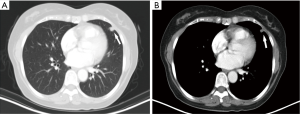
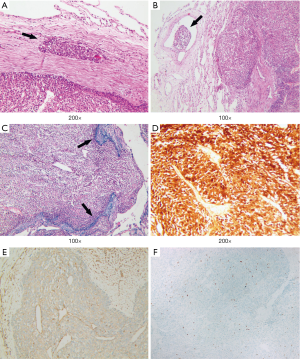
A nodular lesion in the lingular lobe was discovered on a chest CT in a 66-year-old woman with 3-month history of cough. Antibiotic therapy was instituted, and had no effect on the lesion. The physical examination results were normal. All the blood measurements including tumor markers were within normal limits. The chest CT showed a 0.9 cm × 0.8 cm lingular nodular lesion with pleural adhesion (Figure 6A,B). The nodule had ill-defined margins, uneven density and middle enhanced character. There were no swollen lymph nodes in the hilum of the lung or mediastinum. Besides, no bone metastases were detected by 99TcM-MDP bone scintigraphy. A lingular divisionectomy was then performed under VATS. Pathological study revealed a firm, grayish-white tumor measuring 0.9 cm × 0.7 cm × 0.6 cm. The spherical mass had poorly defined margin with visceral pleura invasion. There were no visible invasive or metastatic lesions in the surgical margins or lymph nodes. The tumor cells were heterogeneous with capsular invasion, and tumor embolus, while elastic fiber was positive (Figure 7A,B,C). Microscopically, there was obvious cell atypia, and nuclear divisions were seen. Immunohistochemical staining for S-100 protein and VIM were positive (Figure 7D,E). The tumor cells were negative for CK, EMA, TTF-1, CD56, SYN, CgA, CK7, CK5/6, P40, HMB45, Melan-A, CD3, ACTIN. The total positive rate of Ki-67 was about 1–2%, reaching 4–5% in “hot-spot” regions (Figure 7F). This patient was pathologically diagnosed as a MPGCT based on the morphologic findings. The patient was discharged 5 days after surgery. She was referred to our Pulmonary Department and started chemotherapy. She received pemetrexed (500 mg/m2 on day 1) plus carboplatin [area under the curve (AUC) × (creatinine clearance rate + 25), AUC =5, on day 1]. This treatment was repeated every 21 days for up to 4 cycles. Four years after surgery, the patient remained symptom-free and there was no recurrence of the tumor on the follow-up CT scan.
Study selection
One hundred and twenty-three studies were generated through the search of the relevant databases. After screening studies according to inclusion criteria and exclusion criteria, seventy-eight patients from 43 studies (7-28) were included (29-49). Three current patients from Shanghai Chest Hospital were added, therefore, a total of 81 patients were analyzed.
Demographics and clinical information
Of the 81 patients, 51 were women (63.0%) and 30 were men (37.0%). The average age of all patients was 42.53 years (ranging from 6–84 years). Average age in male patients was 48.8 years (ranging from 6–84 years), and 39.2 years in female patients (ranging from 8–67 years). Among all the patients with PGCT, the Netherlands (31 cases), the United States (24 cases), Japan (7 cases), and China (6 cases) were the most common region. Of the 43 patients with relatively less information, 37 had a known history of smoking.
There were 55 patients (67.9%) with symptoms, while the other 26 patients (32.1%) were asymptomatic. The common symptoms found in patients were: cough (37.0%), dyspnea (13.6%), chest pain (11.1%), fever (11.1%), shortness of breath (8.6%), hemoptysis (8.6%), and chest tightness (2.4%).
Among the 81 patients enrolled in this study, the locations of tumor included trachea, bronchus, and lung tissue, were 21, 38, and 22 cases, respectively. Of which bronchus was the most common site of PGCT, which accounted for 46.9% of all cases. Of the 59 patients with PGCT in trachea or bronchus, 24 (40.67%) had signs of airway obstruction observed from the chest CT image.
There were available data on diagnostic method for 67 patients, among whom 52 were diagnosed by transbronchial biopsy and 15 by surgical biopsy. Of the 81 cases included in this study, 68 were benign PGCT (84.0%), 7 were malignant PGCT (8.6%), while the remaining 6 patients did not have the relevant information. Notably, 18 patients were also co-diagnosed with non-small cell carcinoma or small cell carcinoma.
Treatment and follow-up
Among the 81 patients, 41 received surgical treatment, 12 received bronchoscopic treatment, 28 patients with no relevant information. Besides, 42 patients had no evidence of recurrence at the end of follow-up, 7 patients had residual disease and 8 patients died (non-GCT related) during the follow-up. The demographic and clinical data of benign PGCT (BPGCT), malignant PGCT (MPGCT) and undetermined PGCT (UPGCT) were shown in Table 1.
Table 1
| Clinical features | BPGCT | MPGCT | UPGCT |
|---|---|---|---|
| No. of patients | 68 | 7 | 6 |
| Age (years) | 6–84 (42.5) | 32–66 (53.6) | 9–39 (22.8) |
| Gender | |||
| Female | 43 | 5 | 3 |
| Male | 25 | 2 | 3 |
| Smoker | |||
| Yes | 34 | 2 | 1 |
| No | 3 | 2 | 1 |
| NA | 31 | 3 | 4 |
| Cardinal symptoms | |||
| Cough | 23 | 6 | 0 |
| Dyspnea | 10 | 0 | 1 |
| Chest pain | 8 | 1 | 0 |
| Fever | 7 | 1 | 0 |
| Shortness of breath | 5 | 2 | 0 |
| None | 26 | 0 | 0 |
| Length (cm) | 0.2–8 (1.4)† | 0.9–14 (5.7) | 0.6–4.5 (1.9)‡ |
| Location | |||
| Trachea | 17 | 0 | 4 |
| Bronchus | 34 | 2 | 2 |
| Lung tissue | 17 | 5 | 0 |
| Diagnostic procedure | |||
| TBBX | 43 | 4 | 5 |
| Surgical biopsy | 11 | 3 | 1 |
| NA | 14 | 0 | 0 |
| Treatment | |||
| Surgery | 33 | 6 | 4 |
| Endoscopy | 10 | 0 | 2 |
| Chemotherapy | 0 | 2 | 0 |
| Radiotherapy | 0 | 1 | 0 |
| None | 23 | 0 | 0 |
| NA | 2 | 0 | 0 |
| Outcome | |||
| NER | 34 | 3 | 5 |
| Non-GCT deaths | 8 | 0 | 0 |
| Residual disease | 7 | 0 | 0 |
| Recurrence | 2 | 0 | 1 |
| Lost | 2 | 1 | 0 |
| NA | 16 | 3 | 0 |
†, 9 patients without data; ‡, 2 patients without data. PGCT, pulmonary granular cell tumor; BPGCT, benign PGCT; MPGCT, malignant PGCT; UPGCT, undetermined PGCT; NA, not available; TBBX, transbronchial biopsy; NER, no evidence of recurrence.
Pathological findings
In general, PGCT presents as a firm mass that may be pedunculated or sessile. The tumor tissue has obvious boundaries with surrounding tissues. Histologically, it is often limited, but not encapsulated, and is closely related to the covered skin or mucosa. The tumor cells are polygonal to spindle-shaped with abundant eosinophilic granular cytoplasm and small round, vesicular, or densely chromatic nuclei. Immunohistochemically, 57 cases of PGCT were positive for S-100, 26 cases were positive for PAS, 15 cases were positive for Ki-67, 11 patients were positive for CD68, 10 patients were positive for VIM and 9 patients were positive for NSE. In addition, some patients showed positive SOX10, CD56, PASD, CgA, α1-antitrypsin, α1, antichymotrypsin and inhibin-alpha.
Gene sequencing results
The gene sequencing results were negative in case 2 who was a benign PGCT. No gene mutation or somatic mutation was detected, suggesting that the tumor mutational burden (TMB) was extremely low. However, the gene sequencing results in case 3 who was a malignant PGCT showed a significant increase in WRN, KMT2A, RPA1, NSD1, DDR2, ZNRF3, NOTCH4 (p.Val315Glu) gene expression (abundance >20%). Furthermore, CSF1R, FAT3, NOTCH4 (p.Gly346Asp), GRIN2A, RAD50 gene expression were also increased (Table 2). In addition, high TMB (15.9 mutations per megabase), and microsatellite-stability were also detected.
Table 2
| Gene | Variant type | Exon | cDNA change | Amino acid change | Allelic fraction |
|---|---|---|---|---|---|
| CSF1R | Missense | 4 | c.704T>G | p.Val235Gly | 19.99% |
| DDR2 | Missense | 10 | c.964C>T | p.Leu322Phe | 24.19% |
| FAT3 | Missense | 9 | c.6671A>G | p.Asp2224Gly | 11.43% |
| GRIN2A | Missense | 6 | c.1196G>C | p.Cys399Ser | 15.37% |
| KMT2A | Missense | 27 | c.7262T>A | p.Met2421Lys | 26.05% |
| NOTCH4 | Missense | 6 | c.1037G>A | p.Gly346Asp | 17.05% |
| NOTCH4 | Missense | 6 | c.944T>A | p.Val315Glu | 21.24% |
| NSD1 | Missense | 13 | c.4945G>C | p.Ala1649Pro | 25.50% |
| RAD50 | Missense | 8 | c.1232C>G | p.Ala411Gly | 12.86% |
| RPA1 | Missense | 14 | c.1540A>G | p.Met514Val | 25.51% |
| WRN | Missense | 32 | c.3755T>C | p.Ile1252Thr | 27.88% |
| ZNRF3 | Missense | 8 | c.1903G>C | p.Ala635Pro | 21.61% |
NGS, next generation sequencing.
Discussion
GCT is a distinctly uncommon neoplasm of Schwannian phenotype origin that was first described by Abrikossoff in 1926 (19,50). PGCT is very rare, accounting for about 6–10% of GCT. In this study, we present 3 cases of PGCT, in which two were benign PGCT and one was malignant PGCT. We performed a systematic literature review of 81 cases in total, and only 7 malignant PGCT patients have been reported in the existing literature thus far (7,8,14,21,27,38).
Our results showed that most patients with PGCT were middle-aged women, and the largest number of patients was observed in the Netherlands. Patients with PGCT had a wide range of symptoms, with cough, dyspnea and chest pain being the most common symptoms, while about a third of patients having no symptoms. PGCT can occur in different locations in the lung, including trachea, bronchus and pulmonary tissue. The size of lung lesions varied from 0.2 to 14 cm. Immunohistochemically, 57 cases of PGCT were positive for S-100, suggesting that S-100 has a certain value for the diagnosis of GCT. Nevertheless, no significant differences in immunohistochemistry results were observed between the benign and malignant PGCT. Ki-67 is a useful indicator for identification between the benign and malignant tumors; however, it does not contribute to the identification of the benign from malignant PGCT. PGCT can occur with simultaneous GCT in other organs, and can also occur with other tumors. About one-quarter of PGCT patients had other types of tumors, among which non-small cell lung cancer was the most common. The observed PGCTs in our review were mostly benign, and they had a good prognosis. There is a lack of standard treatment or treatment guideline for PGCT. In practice, surgical resection was the main treatment, and more than half of the patients had no evidence of recurrence after follow-up.
After analyzing the clinical data from 7 patients with MPGCT, we found that most of the patients were women. Microscopic observation of tissue sections of malignant PGCT showed spindle tumor cells, and the tumor cells were pleomorphic with prominent nucleoli, mitotic figures, increased nuclear to cytoplasm ratio and presence of tumor necrosis. Lauro et al. have reported a case of malignant PGCT in which the tumor tissue coexisted with small cell lung cancer. This case was diagnosed as an unresectable tumor due to the specificity of tumor location. After 6 cycles of etoposide and cisplatin regimen chemotherapy and radiotherapy, the disease reached complete response (14). To date, only two other malignant PGCT patients underwent gene sequence as reported by two previous studies. ATM mutations were observed in one case of malignant PGCT. The abnormalities of ASXL1-, NOTCH2-, and PARP4-mediated pathways may have driving mutations in the other case of malignant PGCT (7,8). We conducted an next generation sequencing (NGS) analysis of tumor tissues from the patient with malignant PGCT, and the results showed a significant increase in WRN, KMT2A, RPA1, NSD1, DDR2, ZNRF3, NOTCH4 (p.Val315Glu) gene expression (abundance >20%). Genetic testing may help us to understand the mechanism of malignant PGCT. The gene sequencing results in our study were different from cases in previous studies (7,8). We infer that this may be due to individual and racial differences. Further gene analysis data from malignant PGCT cases are needed for identifying the gene mutations involved in the pathogenesis of malignant PGCT.
This study has some limitations. First, this was a retrospective analysis that has the inherent defects. Secondly, as the data in some reports were not described in detail, some data were missing. Finally, some of the included studies were conducted by radiologists or pathologists, and the data from these studies were biased towards radiological or pathological findings.
Conclusions
In conclusion, PGCT is a rare pulmonary tumor, and is benign in most cases. Surgical resection is often used for treatment and the prognosis tends to be good. It remains unclear which genes are important for the development of PGCT. Gene sequencing of the malignant case revealed increased expression of genes of unknown clinical significance, which need to be further explored.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-133
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-133
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-133). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Chest Hospital (Ethics number: KS2014) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ordóñez NG. Granular cell tumor: a review and update. Adv Anat Pathol 1999;6:186-203. [Crossref] [PubMed]
- Chimelli L, Symon L, Scaravilli F. Granular cell tumor of the fifth cranial nerve: further evidence for Schwann cell origin. J Neuropathol Exp Neurol 1984;43:634-42. [Crossref] [PubMed]
- Kurtin PJ, Bonin DM. Immunohistochemical demonstration of the lysosome-associated glycoprotein CD68 (KP-1) in granular cell tumors and schwannomas. Hum Pathol 1994;25:1172-8. [Crossref] [PubMed]
- Pareja F, Brandes AH, Basili T, et al. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat Commun 2018;9:3533. [Crossref] [PubMed]
- Deavers M, Guinee D, Koss MN, et al. Granular cell tumors of the lung. Clinicopathologic study of 20 cases. Am J Surg Pathol 1995;19:627-35. [Crossref] [PubMed]
- Fanburg-Smith JC, Meis-Kindblom JM, Fante R, et al. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779-94. [Crossref] [PubMed]
- Xu S, Zhao Q, Wei S, et al. Next Generation Sequencing Uncovers Potential Genetic Driver Mutations of Malignant Pulmonary Granular Cell Tumor. J Thorac Oncol 2015;10:e106-9. [Crossref] [PubMed]
- Davis R, Deak K, Glass CH. Pulmonary Granular Cell Tumors: A Study of 4 Cases Including a Malignant Phenotype. Am J Surg Pathol 2019;43:1397-402. [Crossref] [PubMed]
- Desai DP, Maddalozzo J, Holinger LD. Granular cell tumor of the trachea. Otolaryngol Head Neck Surg 1999;120:595-8. [Crossref] [PubMed]
- Al-Ghamdi AM, Flint JD, Muller NL, et al. Hilar pulmonary granular cell tumor: a case report and review of the literature. Ann Diagn Pathol 2000;4:245-51. [Crossref] [PubMed]
- Husain M, Nguyen GK. Cytopathology of granular-cell tumor of the lung. Diagn Cytopathol 2000;23:294-5. [Crossref] [PubMed]
- Abdulhamid I, Rabah R. Granular cell tumor of the bronchus. Pediatr Pulmonol 2000;30:425-8. [Crossref] [PubMed]
- Jin Y, Kuroda N, Kakiuchi S, et al. Bronchial granular cell tumor with osteopontin and osteonectin expression: a case report. Pathol Int 2000;50:421-6. [Crossref] [PubMed]
- Lauro S, Trasatti L, Bria E, et al. Malignant bronchial Abrikossoff's tumor and small cell lung cancer: a case report and review. Anticancer Res 2001;21:563-5. [PubMed]
- Cutlan RT, Eltorky M. Pulmonary granular cell tumor coexisting with bronchogenic carcinoma. Ann Diagn Pathol 2001;5:74-9. [Crossref] [PubMed]
- Muhammad AA, Sikka P, Dhillon RS, et al. Co-existing granular cell tumor and adenocarcinoma of the lung: a case report and review of the literature. Respir Care 2001;46:702-4. [PubMed]
- Amar YG, Nguyen LH, Manoukian JJ, et al. Granular cell tumor of the trachea in a child. Int J Pediatr Otorhinolaryngol 2002;62:75-80. [Crossref] [PubMed]
- Prasad M, Keller JL. Clinical problem solving: pathology. Pathology quiz case 2: granular cell tumor of the trachea. Arch Otolaryngol Head Neck Surg 2002;128:593-594-5. [PubMed]
- van der Maten J, Blaauwgeers JL, Sutedja TG, et al. Granular cell tumors of the tracheobronchial tree. J Thorac Cardiovasc Surg 2003;126:740-3. [Crossref] [PubMed]
- Hosaka T, Suzuki S, Niikawa H, et al. A rare case of a pulmonary granular cell tumor presenting as a coin lesion. Jpn J Thorac Cardiovasc Surg 2003;51:107-9. [Crossref] [PubMed]
- Jiang M, Anderson T, Nwogu C, et al. Pulmonary malignant granular cell tumor. World J Surg Oncol 2003;1:22. [Crossref] [PubMed]
- Ipakchi R, Zager WH, de Baca ME, et al. Granular cell tumor of the trachea in pregnancy: a case report and review of literature. Laryngoscope 2004;114:143-7. [Crossref] [PubMed]
- Stieglitz F, Kitz R, Schafers HJ, et al. Granular cell tumor of the trachea in a child. Ann Thorac Surg 2005;79:e15-6. [Crossref] [PubMed]
- Yoon Y, Curry K. Concurrence of granular cell tumor and Mycobacterium tuberculosis. South Med J 2005;98:1034-5. [Crossref] [PubMed]
- Miyake M, Tateishi U, Maeda T, et al. Bronchial granular cell tumor: a case presenting secondary obstructive changes on CT. Radiat Med 2006;24:154-7. [Crossref] [PubMed]
- Vaos G, Zavras N, Priftis K, et al. Bronchial granular cell tumor in a child: impact of diagnostic delay on the type of surgical resection. J Pediatr Surg 2006;41:1326-8. [Crossref] [PubMed]
- Satoh Y, Okumura S, Nakagawa K, et al. Complete removal of a bronchial granular cell tumor by bronchoplasty. Thorac Cardiovasc Surg 2007;55:465-7. [Crossref] [PubMed]
- Joung MK, Lee YJ, Chung CU, et al. A case of granular cell tumor of the trachea. Korean J Intern Med 2007;22:101-5. [Crossref] [PubMed]
- Bekteshi E, Toth JW, Benninghoff MG, et al. Granular cell tumor of trachea. J Bronchology Interv Pulmonol 2009;16:68-9. [Crossref] [PubMed]
- Shinohara H, Tsuchida M, Hashimoto T, et al. Large bronchial granular cell tumor. Gen Thorac Cardiovasc Surg 2009;57:484-7. [Crossref] [PubMed]
- Kutuya N, Akiduki A. Radiologic appearance of a bronchial granular cell tumor with secondary obstructive changes. Clin Imaging 2010;34:148-51. [Crossref] [PubMed]
- Fang HY, Wu CY, Huang HJ, et al. Granular cell tumor of the lung. Lung 2010;188:355-7. [Crossref] [PubMed]
- Meyer MA, Becker JM, Quinones W. Endobronchial granular cell tumor: a case report. J Radiol Case Rep 2010;4:29-35. [Crossref] [PubMed]
- Zarghouni M. Benign endobronchial granular cell tumor resulting in right middle lobe collapse. Proc (Bayl Univ Med Cent) 2012;25:365-6. [Crossref] [PubMed]
- Sjogren PP, Sidman JD. Use of the carbon dioxide laser for tracheobronchial lesions in children. JAMA Otolaryngol Head Neck Surg 2013;139:231-5. [Crossref] [PubMed]
- Kim HJ, An S, Kim HR. Primary bronchial granular cell tumor in an adult male. Korean J Thorac Cardiovasc Surg 2014;47:193-6. [Crossref] [PubMed]
- Badyal RK, Vasishta RK, Kakkar N, et al. Benign granular cell tumor of the lung in a child--a case report. Fetal Pediatr Pathol 2014;33:191-5. [Crossref] [PubMed]
- Cheng AP, Dong MJ, Fu LP, et al. A rare case of pulmonary malignant granular cell tumor detected with 18F-FDG PET/CT imaging. Clin Nucl Med 2014;39:816-8. [Crossref] [PubMed]
- Guarnieri T, Cardinale L, Macchia G, et al. Multiphasic multidetector computed tomography study of a rare tracheal tumor: granular cell tumor. Case Rep Pulmonol 2014;2014:807430.
- Venur VA, Zhang G, Farver C, et al. Coexistent pulmonary granular cell tumor and adenocarcinoma of the lung. Transl Lung Cancer Res 2014;3:262-4. [PubMed]
- van de Loo S, Thunnissen E, Postmus P, et al. Granular cell tumor of the oral cavity; a case series including a case of metachronous occurrence in the tongue and the lung. Med Oral Patol Oral Cir Bucal 2015;20:e30-3. [Crossref] [PubMed]
- Nath D, Gupta A, Arava S, et al. Synchronous existence of granular cell tumor and small cell carcinoma of lung: An unusual entity. Indian J Pathol Microbiol 2016;59:90-2. [PubMed]
- Meena NK, Jeffus SK, Lindberg MR, et al. How Long Is Too Long? Trials and Tribulations of an Indolent Tumor. J Bronchology Interv Pulmonol 2016;23:242-4. [Crossref] [PubMed]
- Lee DH, Yoon TM, Lee JK, et al. Unusual Granular Cell Tumor of the Trachea Coexisting With Papillary Thyroid Carcinoma and Masquerading as Tracheal Invasion of Recurred Thyroid Carcinoma: A Case Report. Medicine (Baltimore) 2016;95:e3547. [Crossref] [PubMed]
- Esterson YB, Edelman MC, Lipskar AM, et al. A case of bronchial granular cell tumor in a pediatric patient. Clin Imaging 2017;43:15-8. [Crossref] [PubMed]
- Farooqui SM, Khan MS, Adhikari L, et al. Multifocal Pulmonary Granular Cell Tumor Presenting with Postobstructive Pneumonia. Case Rep Pulmonol 2017;2017:8513702.
- Finer EB, Villalba JA, Pitman MB. Granular cell tumor of the lung. Diagn Cytopathol 2019;47:345-6. [Crossref] [PubMed]
- Jobrack AD, Goel S, Cotlar AM. Granular Cell Tumor: Report of 13 Cases in a Veterans Administration Hospital. Mil Med 2018;183:e589-93. [Crossref] [PubMed]
- Ishibashi H, Baba S, Nakashima Y, et al. Endobronchial Granular Tumor Excision With Bronchial Resection Inclusive of Second Carinoplasty. Ann Thorac Surg 2018;105:e193-4. [Crossref] [PubMed]
- Abrikossoff A. Über Myome ausgehend von der quergestreiften willkürlichen Muskulatur. Virchows Archiv 1926;260:215-33. [Crossref]



