Selective estrogen receptor modulators in the prevention of breast cancer in premenopausal women: a review
Introduction
Breast cancer is the second most common type of cancer worldwide and the most common cancer in women (1). It principally affects post-menopausal women but when it is detected in younger patients, the prognosis is worse and a more aggressive behavior is observed. Indeed, higher grade, large tumors and lymph node involvement are more common in premenopausal patients (2). There have been numerous trials of breast cancer treatment in postmenopausal women, 6 articles talking about endocrine therapy were published in 2018 just from the American Society of Clinical Oncology ASCO (3), yet few trials have evaluated the long-term effects of preventive treatment in pre-menopausal women at risk of breast cancer.
The Gail model defines a high risk as having at least one breast biopsy with the presence of pre-cancerous lesions, with a 5-year predicted breast cancer risk >1.66% over the rest of the population, or a first-degree relative with breast cancer (4). The pre-cancerous lesions can be found by mammography, eco-sonogram or biopsy; and some of them are carcinoma in situ, atypical and hyperplastic lesions (5). Atypical hyperplasia (AH), confers an absolute risk that can generally be estimated generally as 1% per year, although a wider disease spread further increases this risk. However, preventive medications reduce breast cancer risk by 70% in AH (6). Other lesions like in situ ductal carcinoma, which now represents 20–25% of all breast cancers, confer a 2% annual risk to develop an invasive disease (7). The increased risk of developing carcinoma associated with these lesions was associated with the ipsi- and contralateral breasts (8).
On the other hand, the most frequent hereditary cause of breast cancer is hereditary breast and ovarian cancer syndrome (HBOC), which is caused by a germline mutation in either of the breast cancer genes (BRCA1 or BRCA2); which are considered to represent 20–25% of the total hereditary factors for breast cancer (9,10). Evidence indicates that specific mutations in other genes also confer increased breast cancer risk, including those in Ataxia-Telangiectasia mutated (ATM), Cadherine-1 (CDH1), Checkpoint kinase 2 (CHEK2), Nibrin gene (NBN), Neurofibromin-1 (NF1), Partner and localizer of BRCA2 (PALB2), Phosphatase and tensin homolog (PTEN), Serine/threonine kinase-11 (STK11), and Tumor protein-53 (TP53) also confer increased breast cancer risk (11).
Another important factor associated with the risk of developing or breast cancer in pre- and post-menopausal women is breast density (12). Controlling breast density can aid risk management, both in a population screening context and in terms of the management and surveillance of women at increased risk of developing breast cancer. In particular, this parameter can help identify populations that might benefit from enhanced surveillance or primary prevention interventions (13).
It is estimated that 11% of the breast cancer patients are pre-menopausal and more than 50% of them express hormone receptors, making them candidates to benefit from endocrine interventions (14). Amongst the endocrine interventions for hormone sensitive breast cancer, three main families exist: aromatase inhibitors (AI), selective estrogen receptor modulators (SERMs) and the recently developed family of selective estrogen receptor down-regulators (SERDs) (15). In terms of AIs, three of them can improve the efficacy of adjuvant endocrine treatment if used instead of, or sequentially, with tamoxifen, either non-steroidal (anastrozole and letrozole) or steroidal (exemestane) AIs. However, the use of AIs is associated with significant adverse events, such as arthralgia, bone pain and osteoporosis (16). The FATA-GIM3 study investigated the schedule and type of AIs to be used as adjuvant treatment for hormone receptor-positive early breast cancer. Accordingly, it was shown that 5-year treatment with AIs was not superior to a 2-year treatment with tamoxifen followed by a 3-year treatment with AIs (17).
Regarding SERDs, fulvestrant is currently only SERD that has been approved for use in humans, a 7α-alkylsulphinyl analogue of 17β estradiol. Fulvestrant is a competitive inhibitor of estradiol binding to the ER, with a binding affinity of 89% (18). Fulvestrant is an oral SERD with a unique method of action. It is a highly specific inhibitor of ERs in the mammary gland, downregulating ERs through inhibition and degradation (19). In preclinical studies AZD9496 also proved to be a potent antagonist that degrades ERs. Recently, a phase I clinical trial of AZD9496 reported it was well tolerated and with an acceptable safety profile (20). GDC-0810, a novel non-steroid SERD, alters the conformation of ERα relative to that induced by currently approved therapeutic agents, suggesting a unique mechanism of action. GDC-0810 has robust in vitro and in vivo activity against a variety of human breast cancer cell lines and patient derived xenografts, including a tamoxifen-resistant model that harbors mutated ERα. Notably, GDC-0810 is currently being evaluated in Phase II clinical studies in women with ER-positive breast cancer (21). SERMs which exert estrogenic and antiestrogen actions, are the most frequently used for breast cancer (22). SERMs regulate transcriptional events in target tissues by acting as agonists and mimicking the effects of estrogen in some tissues. In other tissues, they may act as antagonists by binding to specific ligand binding domains of ERs and inhibiting their biological activity (23). The therapy with SERMs were associated with lower risk of primary invasive ER positive breast cancer (24). Although not all hormone receptor positive breast cancers benefit from treatment with SERMs, they are considered as a classic endocrine therapy for early breast cancers (25). SERMs have been seen to be capable of reducing mammary density and thus, to have breast cancer-preventing activity (26,27). A list of current applications of FDA-approved SERMs and clinical trials is presented in Table 1.
Table 1
| SERM | Indication |
|---|---|
| FDA approved SERMs | |
| Tamoxifen | Reduction of breast cancer incidence in high-risk women (28,29). Treatment of metastatic breast cancer (30) |
| Raloxifene | Prevention/treatment of osteoporosis (31). Breast cancer prevention in post-menopausal women (32) |
| Toremifene | Treatment of metastatic breast cancer in postmenopausal women (33) |
| Clomiphene | Treatment of ovulatory alterations (34) |
| Anordrin | Anti-fertility treatment (35) |
| Bazedoxifene | Prevention/treatment of osteoporosis (36) |
| Broparestrol | Dermatological use. Breast cancer treatment (37) |
| Cyclofenil | Potential ER breast cancer treatment (38) |
| Lasofoxifene | Prevention/treatment of osteoporosis. Treatment of vaginal atrophy (39) |
| Ormeloxifene | Oral contraceptive. Dysfunctional uterine bleeding treatment (40) |
| Ospemifene | Dyspareunia treatment (41) |
| Clinical trials | |
| Acolbifene | Phase III clinical trials for breast cancer treatment (42) |
| Endoxifene | Hormone sensitive breast cancer treatment (43) |
| Afimoxifene (4 hydroxytamoxifen) | Treatment of hyperplasia. Phase II clinical trial for breast cancer prevention (44) |
| Elacestrant | Menopausal symptoms. Hormone sensitive breast cancer treatment (45) |
| Enclomiphene | Treatment of male hypogonadism (46) |
Several authors have reported the use of SERMs as preventive treatment for breast cancer. For instance, administration of tamoxifen (the first generation of SERMs) for 5 years in a primary prevention setting decreased the risk of invasive breast cancer by approximately 30–40% (47). In a randomized phase III trial with a long-term follow-up, and in comparison to a systemically untreated control group, adjuvant treatment with tamoxifen for 2 years, resulted in a long-term reduction of breast cancer–related mortality in pre-menopausal patients with ER-positive breast cancer (48). Also, the results of the STAR study revealed raloxifene (a second-generation SERM) produced a substantial decrease in breast cancer risk in post-menopausal patients, although its performance was inferior to tamoxifen (49). Nevertheless, as a preventive strategy, raloxifene has a better safety profile (lower risk of endometrial cancer, less thromboembolic effects and fewer cataracts and cataract surgeries) (50). In a phase II trial, tamoxifen was compared to raloxifene and a placebo in pre-menopausal women with ER-positive breast cancer (51). It was concluded that tumor cell proliferation in pre-menopausal breast cancer patients was not reduced by a low weekly dose of tamoxifen or a standard dose of raloxifene. However, extensive modulation of Ki-67 was observed in the tamoxifen arm of patients with high CYP2D6 expression. Likewise, discontinuation of tamoxifen-based therapy was associated with changes in mammary density, with a mean increase of 1.8% (52). Acolbifene, and its pro-drug EM-800 (a fourth generation SERM), have been associated with growth inhibition of tumor xenografts. The lack of estrogen agonist activity of EM-800 in the uterus and its proven activity in tamoxifen-resistant metastatic diseases, make EM-800 an attractive agent for the treatment and prevention of ER-positive breast cancer (53). Regarding the use of SERMs as preventive agents in breast cancer, a review published in 2009 compared the effectiveness and the efficacy of medications to reduce the risk of primary breast cancer in women at risk. The review concluded that the efficacy of tamoxifen citrate (RR 0.70; 95% CI, 0.59 to 0.82; 4 trials) and raloxifene (RR 0.44; 95% CI, 0.27 to 0.71; 2 trials) reduced the risk of invasive breast cancer in women compared to a placebo by 0.7% to 1.0% per year (54,55).
Adverse effects caused by SERMs limit their general preventive use and there are several records of such events, including venous thromboembolic events, life-threatening pulmonary embolism and an increase of endometrial cancer (56). To date, the impact of SERMs in breast cancer prevention in pre-menopausal women at high risk of developing hormone sensitive breast cancer has yet to be thoroughly reviewed. Considering the multiple benefits associated with preventative therapy and the early management of hormone sensitive breast cancer, a systematic review of the current literature has been carried out to evaluate the preventive benefits of different SERMs in this scenario.
Aim
The aim of this review was to analyze the most recent and complete studies that evaluate the effectiveness and safety of SERMs in pre-menopausal women at high risk of developing hormone-sensitive breast cancer.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-1956).
Methods
This study was designed according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (57).
Eligibility criteria
Original articles from 2008 to 2018 that reported randomized controlled trials that involve an intervention group in which SERMs were administered to pre-menopausal women at risk of developing hormone sensitive breast cancer were included in this study. Articles not fulfilling all these criteria were excluded.
Information sources
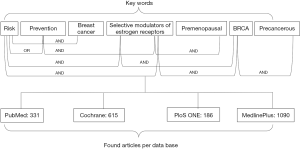
Different databases were searched for these articles, including PubMed, MedlinePlus, PLoS One, Cochrane Breast Cancer Specialized Register, Clinical Trials.gov, American Society of Clinical Oncology (58) symposium, European Society for Medical Oncology (ESMO). Relevant studies were obtained from Pubmed, MedlinePlus, PLoS One and Cochrane.
Search strategy
The titles and abstracts or the full articles in the PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Cochrane (http://www.cochranelibrary.com), PLoS One (http://journals.plos.org/plosone/) and MedlinePlus (http://www.ncbi.nlm.nih.gov/pubmed) databases were searched using the following search terms in titles and abstracts: risk, precancerous, prevention, BRCA, premenopausal, Selective Modulators of Estrogen Receptors (SERMs), AND breast cancer (a diagram of the search algorithm is shown in Figure 1). No language restriction was applied to the literature search and the search was limited to studies in humans. Two reviewers (ADN and AT) evaluated each article independently.
Study selection
Original studies were included if they met the following criteria: (I) interventional studies (employing either a parallel or cross-over design), post hoc analyses and observational studies (employing either a prospective or retrospective design); (II) studies of the impact of SERMs on women at risk of developing breast cancer; and (III) presentation of sufficient information on treatment, antigen Ki67, the development of breast lesions, the follow-up in each group or providing the net change in values. Exclusion criteria were: (I) lack of sufficient information on the baseline parameters, or of the follow-up to treatment, antigen Ki67 or the development of breast lesions.
Data collection
Eligible studies were reviewed and the following data were extracted: (I) first author’s name; (II) year of publication; (III) study site; (IV) study design; (V) inclusion criteria and underlying disease; (VI) number of participants; (VII) age, menarche status, parity; (VIII) baseline, follow-up, change in Ki67 values and development of breast lesions.
Risk of bias
A systematic assessment of the bias in the studies included was performed by applying the Cochrane criteria (59). The items used to assess each study were: adequacy of sequence generation, allocation concealment, blinding, drop-outs addressed (incomplete outcome data), selective outcome reporting, and other potential sources of bias. An evaluation of low, high or unclear risk of bias was assigned to each study, according to the recommendations of the Cochrane handbook. By labeling an item as “unclear”, it was attributed an unclear or unknown risk of bias. The risk of bias assessment was performed independently by two reviewers.
Data analysis
We analyzed the results of eligible trials to obtain more precise estimates of the major health outcomes. Standard deviations (SDs) of the mean difference were calculated using the following equation:
where SDpre and SDpost are the standard deviations at the pre-treatment and post-treatment stages, respectively, and we assumed a correlation coefficient R=0.5. If the outcome measurements were reported as the median and interquartile range, mean and SD values were estimated using the method described by Hozo et al. (60). The risk ratios (rate ratio, hazard ratio, or relative risk) from each trial were estimated and used them as the effect measures (55,61).
Results
Flow chart and characteristics of the studies included
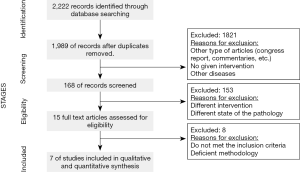
A flow chart of the selection process followed is shown in Figure 2. The articles whose titles and/or abstracts were irrelevant in the context of this review were discarded through the initial screening. Among the 15 full-text articles initially considered to be eligible, 8 studies were excluded for the following reasons: they included post-menopausal patients and/or different treatments, they were published before 2008, they were reviews, and/or they dealt with advanced breast cancer.
After the final assessment, only 7 studies met the study criteria and were included in the systematic review (51,53,62-66). Two studies involved the use of two treatments and as each treatment could be analyzed separately, these studies were divided to assess the treatment outcomes with different SERMs independently (51,63). A total of 1,139 participants were involved in the 7 studies examined, in which the number of participants ranged from 14 to 672. In accordance with the inclusion criteria, the studies were all published between 2008 and 2018, and they were conducted in the United States, Brazil, Canada and Italy. Other important characteristics of the studies included are summarized in Table 2.
Table 2
| Author | Year | Country | Design | Duration | Participants | Age, mean, SD | SERMs |
|---|---|---|---|---|---|---|---|
| Fabian (53) | 2015 | USA | Pilot study | 6–8 months | 25 | 42.8 (5.2) | Acolbifene 20 mg per day |
| Lima (62) | 2012 | Brazil | * | 22 days | 40 | 25.94 (1.41) | Raloxifene 60 mg per day |
| Lucato (Tam) (63) | 2015 | Brazil | * | 22 days | 16 | 22.31 (6.07) | Tamoxifen 20 mg per day |
| Lucato (Ral) (63) | 2015 | Brazil | * | 22 days | 14 | 25.29 (6.51) | Raloxifene 60 mg per day |
| Eng-Wong (64) | 2008 | USA | Phase II trial | 2 years | 37 | 43 (6.3) | Raloxifene 60 mg per day |
| Bramwell (65) | 2010 | Canada | ** | 5 years | 672 | 45 (6.7) | Tam/placebo 20mg per day |
| Serrano (Tam) (51) | 2013 | Italy | *** | 6 weeks | 50 | 44 (2.51) | Tamoxifen 10 mg per week |
| Serrano (Ral) (51) | 2013 | Italy | *** | 6 weeks | 50 | 46 (3.2) | Raloxifene 60 mg per day |
| Decensi (66) | 2009 | Italy | **** | 2 years | 235 | Undefined | Tamoxifen 5 mg per day |
*, randomized, double-blind study; **, randomized placebo controlled study; ***, three-arm randomized double-blind clinical trial; ****, randomized, double-blind, placebo-controlled trial with a 2×2 factorial design.
The risk of bias (Table 3) was presented on 2 of the 7 studies, because there was no control arm, only reporting the analysis of the treatment group. Nevertheless, the other 5 studies did not present any risk because of they were randomized, double blind type of studies.
The Ki-67 antigen was analyzed before treatment and at the treatment’s end point in just 3 of the 7 studies, obtaining results that reached statistical significance (P<0.001) in only 2 studies. In the study by Lucato et al. (63), the Ki-67 antigen was not measured before treatment, yet the values obtained after treatment were not significantly different between the two groups. Likewise, the results presented in the study by Serrano et al. were also not significant (P=0.78), whereas the studies by Eng-Wong et al., Decensi et al. and Bramwell et al. (64-66) did not evaluate the Ki-67 antigen. In 3 of the selected studies, the breast density was evaluated in mammograms and no major differences in the relative mammograph density were observed following raloxifene therapy (64) when assessed by two different radiologists 1 (P=0.93, P=0.86) and 2 years (P=0.58, P=0.05) after the onset of treatment. However, MRI was also used to measure the breast volume in this study and important differences were evident after the 1st (P=0.0017) and 2nd year (P=0.0004) of treatment. In a study of acolbifene (53), no significant changes in the mammary density were observed after 9 months of treatment (P=0.067). However, a 20% reduction of the mammary density was detected after a 2-year treatment (P=0.003) when the effects of tamoxifen were evaluated (66: see Table 4).
Table 4
| Study | Ki-67 no treatment | Ki-67 post treatment | P | SD of the mean difference | Mammographic density | Volume by MRI | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean pretreatment | Mean post-treatment | Pretreatment to 1st year | Pretreatment to 2nd year | ||||||
| Ki-67 expression | |||||||||
| Acolbifene (Fabian et al.) (53) | 6.6±4.8 | 2.1±1.9 | <0.001 | 4.186 | |||||
| Raloxifene (Lima et al.) (62) | 22.16±1.9 | 2.161±0.181 | <0.001 | 1.905 | |||||
| Tamoxifen (Lucato et al.) (63) | Undefined | 2.02±1.09 | 0.205 | Undefined | |||||
| Raloxifene (Lucato et al.) (63) | Undefined | 3.13±3.23 | 0.205 | Undefined | |||||
| Raloxifene (Eng-Wong et al.) (64) | Undefined | Undefined | Undefined | Undefined | |||||
| Tamoxifen (Bramwell et al.) (65) | Undefined | Undefined | Undefined | Undefined | |||||
| Tamoxifen (Serrano et al.) (51) | 18±4.47 | 19.5±4.41 | 0.78 | 3.027 | |||||
| Raloxifene (Serrano et al.) (51) | 21.5±4.18 | 21±2.16 | 0.78 | 3.027 | |||||
| Tamoxifen (Decensi et al.) (66) | Undefined | Undefined | Undefined | Undefined | |||||
| Breast density by imaging | |||||||||
| Raloxifene (64) | 39% [7–78] | 1 year: 1% (−3 to +5) P=0.86; 2 years: 1% (−2 to +5) P=0.05 |
−17% (−28 to −9) P=0.0017 | −16% (−57 to 25) P=0.0004 | |||||
| Acolbifene (53) | 35.8% (2.9–76.3) | 9 months: −11% (−5%±30%) P=0.067 | Undefined | Undefined | |||||
| Tamoxifen (Decensi) (66) | 49.9% (45.4–54.4) | 12 months: −9.9% (−16.2 to −3.6) 24 months: −16.2% (−22.6 to −9.8) P=0.003 |
Undefined | Undefined | |||||
Values given as the mean ± standard deviation.
Only 3 of the 7 studies reported the frequency of adverse events indicated by the patients. These events were mainly gynecological, such as hot flushes, irregular menses and amenorrhea, or neurological, such as headache, mood alterations and dizziness (Table 5).
Table 5
| Adverse events | Acolbifene (Fabian et al.) (53) | Raloxifene (Eng-Wong et al.) (64) | Tamoxifen (Bramwell et al.) (65) |
|---|---|---|---|
| Hot flushes | 16% | 57% | 82% |
| Irregular menses | 32% | 86% | |
| Amenorrhea | 67% | ||
| Dizziness | 16% | ||
| Muscle cramps | 25% | ||
| Diarrhea | 16% | ||
| Myalgias | 64% | 17% | |
| Vaginal discharge | 11% | 27% | |
| Headache | 25% | 12% | |
| Mood alterations | 32% | ||
| Breast pain | 25% |
Discussion
To the best of our knowledge, this systematic review is the first to compile the evidence obtained from diverse studies that evaluate the use of SERMs in pre-menopausal patients with high breast cancer risk, including early breast cancer or in situ lesions.
According to the National Comprehensive Cancer Network (NCCN) guidelines, there are 3 important agents that can reduce the risk of developing breast cancer. For pre-menopausal women at a high risk of developing breast cancer the gold standard therapy is tamoxifen, a daily dose of 20 mg for 5 years reducing risk by 49% to 86% (67). Data regarding the risk reduction associated with the use of raloxifene at a daily dose of 60 mg is limited to post-menopausal women and it is considered to be inappropriate for pre-menopausal women unless as a part of a clinical trial (68). The final agents indicated are AIs and specifically, exemestane and anastrozole, the use of both limited to post-menopausal women except when part of a clinical trial for pre-menopausal women. Indeed, to date the FDA has not approved the use of these drugs to reduce the risk of breast cancer (69,70).
It is important to highlight that several studies have analyzed different combinations of drugs with hormonal therapy to treat hormone sensitive breast cancer. For example, the HOBOE-2 phase III trial that included 1,065 patients, analyzed the role of AIs and zoledronic acid as an adjuvant treatment of pre-menopausal endocrine-responsive breast cancer. The conclusion of this study was that in pre-menopausal early breast cancer patients, the combination of zolendronic acid and triptorelin is more effective than that of tamoxifen and triptorelin in terms of disease-free survival (DFS) (71). In another study (72), 694 patients were analyzed and the overall survival among post-menopausal patients with hormone receptor-positive metastatic breast cancer who had been randomly assigned to receive the AI anastrozole along with the SERD, fulvestrant, as a first-line therapy was compared to that of patients who received anastrozole alone. The authors concluded that the combination of fulvestrant and anastrozole was associated with enhanced long-term survival compared to anastrozole alone, despite the substantial crossover to fulvestrant after progression during therapy with anastrozole alone (72). The PALOMA 1 study analyzed the safety and efficacy of palbociclib (cyclin dependent kinase 4/6 inhibitor) in combination with letrozole as a first-line treatment in patients with advanced ER-positive, HER2-negative breast cancer. This phase II study concluded that the addition of palbociclib to letrozole significantly improved the progression-free survival (PFS) in women with advanced ER-positive and HER2-negative breast cancer (73). Subsequently, the phase III PALOMA 3 study was carried out to analyze the PFS of 521 patients, indicating that the median PFS was 9.5 months (95% CI) in the fulvestrant plus palbociclib group and 4.6 months in the fulvestrant plus placebo group (95% CI, P<0.0001). The conclusions were that palbociclib combined with fulvestrant resulted in a longer PFS than fulvestrant alone (74). All these studies are searching for the perfect combination of drugs that are likely to be effective against ER positive breast cancer and that could improve the overall survival of these patients.
Nevertheless, SERMs are still the most commonly used hormone therapy, with tamoxifen and raloxifene the most commonly tested SERMs in trials, with acolbifene studied in only one trial. The efficacy of tamoxifen in lowering breast cancer risk was confirmed in a long-term follow-up of the main chemoprevention trials (75,76). Moreover, numerous preclinical studies have investigated the anti-proliferative effects of tamoxifen (77,78). Alternatively, raloxifene has been shown to be effective in breast cancer prevention, the most important effect of which was to decrease breast volume evaluated by MRI (79). Notably, it also has an overall better toxicity profile but weaker activity against intraepithelial lesions (80). Based on the known properties of SERMs, research into these drugs has become more intense in recent years, diversifying the types of patient studied and expanding the horizons of the population that can benefit from their use. Indeed, it is known that they can reduce ERα expression to levels similar to those found in non-neoplastic breast tissue, and decreased the mortality due to breast cancer up to 25–30% (81).
In the studies selected here, modulation of the Ki-67 risk biomarker was often investigated as one of the main outcomes, not least because its reduction is associated with a superior recurrence-free survival and a prognostic factor to determine the patients’ status after chemotherapy in short and long-term follow-up (82). Two of the studies analyzed reported a significant change in the mean Ki-67 expression following treatment with acolbifene (53) and with raloxifene (62). Interestingly, while Ki-67 was not measured at the pre-treatment stage in the study carried out by Lucato et al. (63), its post-treatment expression was low and there were no significant differences between the raloxifene and tamoxifen therapies tested. In general, the results of the studies analyzed so far reveal that acolbifene, raloxifene and tamoxifen each produce an important anti-proliferative effect. Another important factor is the change in mammary density during treatment, which was evaluated in 3 of the studies selected. Interestingly, while 3 different treatments appeared to alter breast density, only tamoxifen produced a significant change in this parameter when the pre-treatment values were compared with those after 2 years of treatment (66), reducing breast density by 20%.
It is important to note that although the selected articles had the same goal, to assess the beneficial effect of SERMs in pre-menopausal women with a high risk of developing breast cancer, different variables were measured. Thus, while some evaluated Ki-67 expression, others analyzed the breast density and its characteristics before and after treatment. This variability in the parameters measured could affect our true understanding of the effectiveness of SERMs as preventative agents. The correct identification of pre-menopausal patients at high risk of breast cancer is also an important aspect of the disease history that could potentially have a meaningful influence on the incidence and mortality rates.
Despite the small body of studies interested in demonstrating beneficial effects of SERMs on the risk of ER positive breast cancer, it was possible to establish the importance of preventive treatment with SERMs in pre-menopausal women. This systematic review provides valuable information regarding the possibility of designing new prevention trials with SERMs, the results of which could help select an appropriate preventive treatment, with major benefits and minimal toxicity.
Conclusions
This systematic review found that adjuvant treatment with SERMs reduces the risk of developing breast cancer, modulating Ki-67 expression and breast density in pre-menopausal women with a high risk of developing early breast cancer. The data obtained also highlighted the need for more systematic trials of the therapeutic use of SERMs in pre-menopausal women with a high risk of breast cancer. Such studies should assess all the critical variables and factors that might influence the risk of developing breast cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-1956
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1956). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GLOBOCAN. GCO. Cancer incidence, mortality and prevalence worldwide. 2018 Available online: http://gco.iarc.fr/
- Wang Y, Lewin N, Qaoud Y, et al. The oncologic impact of hormone replacement therapy in premenopausal breast cancer survivors: a systematic review. Breast 2018;40:123-30. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 2019;37:423-38. [Crossref] [PubMed]
- Pinsky PF, Miller E, Heckman-Stoddard B, et al. Use of raloxifene and tamoxifen by breast cancer risk level in a Medicare-eligible cohort. Am J Obstet Gynecol 2018;218:606.e1-9. [Crossref] [PubMed]
- Chandanwale SS, Kaur S, Nair R, et al. Precancerous breast lesions in benign breast lesions: Review of 430 benign breast lesions. Clin Cancer Investig J 2017;6:30-4. [Crossref]
- Degnim AC. Breast atypia as a biomarker of risk. Curr Breast Cancer Rep 2019; [Crossref]
- van Seijen M, Lips EH, Thompson AM, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer 2019;121:285-92. [Crossref] [PubMed]
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher than average risk: recommendations from the ACR. J Am Coll Radiol 2018;15:408-14. [Crossref] [PubMed]
- Padamsee TJ, Wills CE, Yee LD, et al. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res 2017;19:34. [Crossref] [PubMed]
- Bayraktar S, Arun B. BRCA mutation genetic testing implications in the United States. Breast 2017;31:224-32. [Crossref] [PubMed]
- Blazer KR, Slavin T, Weitzel JN. Increased reach of genetic cancer risk assessment as a tool for precision management of hereditary breast cancer. JAMA Oncol 2016;2:723-4. [Crossref] [PubMed]
- Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol 2011;29:2985-92. [Crossref] [PubMed]
- Duffy SW, Morrish O, Allgood PC, et al. Mammographic density and breast cancer risk in breast screening assessment cases and women with a family history of breast cancer. Eur J Cancer 2018;88:48-56. [Crossref] [PubMed]
- Pistelli M, Mora AD, Ballatore Z, et al. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr Oncol 2018;25:e168-75. [Crossref] [PubMed]
- Nasrazadani A, Thomas RA, Oesterreich S, et al. Precision medicine in hormone receptor-positive breast cancer. Front Oncol 2018;8:144. [Crossref] [PubMed]
- Walker AJ, West J, Card TR, et al. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood 2016;127:849-57. [Crossref] [PubMed]
- De Placido S, Gallo C, De Laurentiis M, et al. Adjuvant Anastrozole versus Exemestane versus Letrozole, upfront or after 2 years of Tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet 2018;19:474-85. [Crossref] [PubMed]
- Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 2004;90:S2-6. [Crossref] [PubMed]
- Blackburn SA, Parks RM, Cheung KL. Fulvestrant for the treatment of advanced breast cancer. Expert Rev Anticancer Ther 2018;18:619-28. [Crossref] [PubMed]
- Nardone A, Weir H, Delpuech O, et al. The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance. Br J Cancer 2019;120:331-9. [Crossref] [PubMed]
- Joseph JD, Darimont B, Zhou W, et al. The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. eLife 2016;5:e15828. [Crossref] [PubMed]
- Bandolia H, Khan W. A review on selective estrogen receptor modulators. RPHS 2019;5:179-81. [Crossref]
- Valéra MC, Fontaine C, Dupuis M, et al. Towards optimization of estrogen receptor modulation in medicine. Pharmacol Ther 2018;189:123-9. [Crossref] [PubMed]
- Nelson HD, Fu R, Zakher B, et al. Medication use for the risk reduction of primary breast cancer in women: updated evidence report and systematic review for the US preventive services task force. JAMA 2019;322:868-86. [Crossref] [PubMed]
- Chinese Anti-Cancer Association. Efficacy of Tamoxifen versus Toremifene in CYP2D6 IM/PM of premenopausal patients with ER-positive early breast cancer. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03351062
- Johansson H, Bonanni B, Gandini S, et al. Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose Tamoxifen and Fenretinide. Breast Cancer Res Treat 2013;142:569-78. [Crossref] [PubMed]
- Isfoss BL, Holmqvist BO, Sand E, et al. Stellate cells and mesenchymal stem cells in benign mammary stroma are associated with risk factors for breast cancer – an observational study. BMC Cancer 2018;18:230. [Crossref] [PubMed]
- Kim JW, Gautam J, Kim JE, et al. Inhibition of tumor growth and angiogenesis of Tamoxifen-resistant breast cancer cells by Ruxolitinib, a selective JAK2 inhibitor. Oncol Lett 2019;17:3981-9. [PubMed]
- Hussein AA, Janabi A, Ali H, et al. Tamoxifen: from anti-cancer to antifungal drug. Intl J Med Rev 2019;6:88-91. [Crossref]
- Cuzick J, Sestak I, Forbes JF, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet 2020;395:117-22. [Crossref] [PubMed]
- Chen LR, Ko NY, Chen KH. Medical treatment for osteoporosis: from molecular to clinical opinions. Int J Mol Sci 2019;20. [PubMed]
- Khorsand I, Kashef R, Ghazanfarpour M, et al. The beneficial and adverse effects of raloxifene in menopausal women: A mini review. J Menopausal Med 2018;24:183-7. [Crossref] [PubMed]
- Shien T, Doihara H, Sato N, et al. Serum lipid and bone metabolism effects of Toremifene vs. Letrozole as adjuvant therapy for postmenopausal early breast cancer patients: results of a multicenter open randomized study. Cancer Chemother Pharmacol 2018;81:269-75. [Crossref] [PubMed]
- Francisco R, Jaroudi S, Murtaza Ali M, et al. Clomiphene for hypogonadism complicated by polycythemia. Proc (Bayl Univ Med Cent) 2019;32:75-7. [Crossref] [PubMed]
- Gu W, Xu W, Sun X, et al. Anordrin eliminates Tamoxifen side effects without changing its antitumor activity. Sci Rep 2017;7:43940. [Crossref] [PubMed]
- Fabian CJ, Nye L, Powers KR, et al. Effect of Bazedoxifene and conjugated estrogen (Duavee®) on breast cancer risk biomarkers in high risk women: A pilot study. Cancer Prev Res (Phila) 2019;12:711-20. [Crossref] [PubMed]
- Hassan M. Synthesis of broparestrol using palladium-catalyzed cross-coupling. J Organ Chem 1987;321:119-21. [Crossref]
- Kelly PM, Keely NO, Bright SA, et al. Novel selective estrogen receptor ligand conjugates incorporating Endoxifen-Combretastatin and Cyclofenil-Combretastatin hybrid scaffolds: synthesis and biochemical evaluation. Molecules 2017;22:1440. [Crossref] [PubMed]
- Crane K. Lasofoxifene reduces breast cancer risk in postmenopausal osteoporotic women. JNCI 2010;102.
- Cardoso PM. A comparative study of the efficacy of Ormeloxifene and Norethisterone in perimenopausal dysfunctional uterine bleeding and perimenopausal symptoms. JDMS 2016;15:57-62.
- Del Pup L. Ospemifene: a safe treatment of vaginal atrophy. Eur Rev Med Pharmacol Sci 2016;20:3934-44. [PubMed]
- Gauthier S, Cloutier J, Dory YL, et al. Synthesis and structure-activity relationships of analogs of EM-652 (Acolbifene), a pure selective estrogen receptor modulator. Study of nitrogen substitution. J Enzyme Inhib Med Chem 2005;20:165-77. [Crossref] [PubMed]
- Ahmad A, Shahabuddin S, Sheikh S, et al. Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther 2010;88:814-7. [Crossref] [PubMed]
- Benante KA, Xu Y, Tull MB, et al. A phase IIB pre-surgical trial of oral Tamoxifen (TAM) versus transdermal 4-hydroxytamoxifen (4-OHT) in women with DCIS of the breast. J Clin Oncol 2018;36.
- Elacestrant monotherapy vs. standard of care for the treatment of patients with ER+/HER2- advanced breast cancer following CDK4/6 inhibitor therapy: a phase 3 randomized, open-label, active-controlled, multicenter trial. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03778931
- Earl JA, Kim ED. Enclomiphene citrate: A treatment that maintains fertility in men with secondary hypogonadism. Expert Rev Endocrinol Metab 2019;14:157-65. [Crossref] [PubMed]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [Crossref] [PubMed]
- Ekholm M, Bendahl P, Fernö M, et al. Two years of adjuvant Tamoxifen provides a survival benefit compared with no systemic treatment in premenopausal patients with primary breast cancer: long-term follow-Up (> 25 years) of the phase III SBII:2 pre trial. J Clin Oncol 2016;34:2232-8. [Crossref] [PubMed]
- Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727.41.
- DeCensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol 2011;22:582-7. [Crossref] [PubMed]
- Serrano D, Lazzeroni M, Gandini S, et al. A randomized phase II presurgical trial of weekly low-dose tamoxifen versus raloxifene versus placebo in premenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res 2013;15:R47. [Crossref] [PubMed]
- Kim WH, Cho N, Kim YS, et al. Mammographic density changes following discontinuation of tamoxifen in premenopausal women with oestrogen receptor-positive breast cancer. Eur Radiol 2018;28:3176-84. [Crossref] [PubMed]
- Fabian CJ, Kimler BF, Zalles CM, et al. Clinical trial of Acolbifene in premenopausal women at high risk for breast cancer. Cancer Prev Res (Phila) 2015;8:1146-55. [Crossref] [PubMed]
- Nelson H, Fu R, Griffin J, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med 2009;151:703-15. [Crossref] [PubMed]
- Nelson H, Fu R, Humphrey L, et al. Comparative effectiveness of medications to reduce risk of primary breast cancer in women. AHRQ Comparative Effectiveness Reviews 2009;217. [PubMed]
- Stubert J, Dieterich M, Gerber B. Medical prevention of breast cancer. Breast Care 2014;9:391-6. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Gonzalez I, Rivera MC, Velazquez DA, et al. Selective estrogen receptor modulators regulate dendritic spine plasticity in the hippocampus of male rats. Neural Plasticity 2012;309494. [PubMed]
- The Cochrane handbook for systematic reviews of interventions. Version 5.1.0; 2011.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:1-10. [Crossref] [PubMed]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005;97:1652-62. [Crossref] [PubMed]
- Lima MA, Borges da Silva B. Expression of Ki-67 and Bcl-2 biomarkers in normal breast tissue from women of reproductive age treated with raloxifene. Arch Gynecol Obstet 2012;285:223-7. [Crossref] [PubMed]
- Lucato MT, Freitas R, Moreira M, et al. Effect of Tamoxifen and Raloxifene on the proliferative activity of the breast epithelium in premenopausal women. Clin Med Insights 2015;9:25-30. [PubMed]
- Eng-Wong J, Orzano-Birgani J, Chow CK, et al. The effect of Raloxifene on mammographic density and breast MRI in premenopausal women at increased risk for breast cancer. Cancer Epidemiol Biomarkers Prev 2008;17:1696-701. [Crossref] [PubMed]
- Bramwell VH, Pritchard KI, Tu D, et al. A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada—Clinical Trials Group Trial, MA.12). Ann Oncol 2010;21:283-90. [Crossref] [PubMed]
- Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized Double-Blind 2 2 Trial of Low-Dose Tamoxifen and Fenretinide for Breast Can. J Clin Oncol 2009;27. [PubMed]
- King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA 2001;286:2251-6. [Crossref] [PubMed]
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol 2011;29:2327-33. [Crossref] [PubMed]
- Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of Exemestane after two to three years of Tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004;350:1081-92. [Crossref] [PubMed]
- Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of Letrozole in postmenopausal women after five years of Tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793-802. [Crossref] [PubMed]
- Perrone F, De Laurentiis M, De Placido S, et al. The HOBOE-2 multicenter randomized phase III trial in premenopausal patients with hormone-receptor positive early breast cancer comparing triptorelin plus either tamoxifen or letrozole or letrozole + zoledronic acid. Ann Oncol 2018;29.
- Mehta RS, Barlow WE, Albain KS, et al. Overall survival with Fulvestrant plus Anastrozole in metastatic breast cancer. N Engl J Med 2019;380:1226-34. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Turner NC, Ro J, André FPALOMA3 study group, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Cuzick J, Decensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. . Lancet Oncol 2011;12:496-503. [Crossref] [PubMed]
- Keilty D, Buchanan M, Ntapolias K, et al. RSF1 and not Cyclin D1 gene amplification may predict lack of benefit from adjuvant Tamoxifen in high-risk pre-menopausal women in the MA.12 randomized clinical trial. PLoS One 2013;8. [PubMed]
- Chun-Yu L, Man-Hsin H, Duen-Shian W, et al. Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A–dependent phospho-Akt inactivation in estrogen receptor–negative human breast cancer cells. BCR 2014;16:431. [Crossref] [PubMed]
- Liu CY, Hung MH, Wang DS, et al. Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A–dependent phospho-Akt inactivation in estrogen receptor–negative human breast cancer cells. BCR 2014;16:431. [Crossref] [PubMed]
- Delille JP, Slanetz PJ, Yeh ED, et al. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging--initial observations. Radiology 2005;235:36-41. [Crossref] [PubMed]
- Gizzo S, Saccardi C, Patrelli TS, et al. Update on Raloxifene: mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet Gynecol Surv 2013;68:467-81. [Crossref] [PubMed]
- Moquitul H, Kartiki V. Pathways to endocrine therapy resistance in breast cancer. Front Endocrinol 2019;10:573. [Crossref] [PubMed]
- Moazed V, Jafari E, Kalantari K, et al. Prognostic significance of reduction in Ki67 index after neoadjuvant chemotherapy in patients with breast cancer in Kerman between 2009 and 2014. Iran J Pathol 2018;13:71-7. [Crossref] [PubMed]