The efficacy of ado-trastuzumab emtansine in patients with ERBB2-aberrant non-small cell lung cancer: a systematic review
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases and is associated with a 5-year survival rate of 15% (1). ERBB2, as a known proto-oncogene, was originally observed during oncogenic activation in breast cancer. Trastuzumab, a monoclonal antibody against ERBB2, has been recommended as the first-line treatment combined with pertuzumab plus docetaxel for ERBB2-positive metastatic breast cancer (2). ERBB2 is also a potential oncogenic cause of NSCLC. Recent literatures revealed that the frequency of ERBB2 gene mutation, gene amplification, protein overexpression in NSCLC were 1–4% (3-7), 2–5% (8-10) and 10–15% (11,12), respectively. However, the exact efficacy of trastuzumab monotherapy was disappointing with an overall response rate (ORR) of 0% (0/10), in patients with diverse ERBB2 alterations (13). Trials of trastuzumab combined with chemotherapy in patients with ERBB2-mutated lung cancer resulted in a response rate up to 50% (14-16). For ERBB2-overexpressed and EGFR-mutant NSCLC, trastuzumab combined with chemotherapy exhibited an ORR of 67% in those with an ERBB2 immunohistochemistry (IHC) status of 3+ (17). ERBB2 pathway activation might contribute differently to the pathogenesis of lung cancer, and in turn, therapies targeting ERBB2 could lead to distinct outcomes (18). As a novel ERBB2-targeted agent, ado-trastuzumab emtansine (T-DM1) is an antibody-drug conjugate composed of trastuzumab and the cytotoxic agent mertansine. T-DM1 binds to ERBB2-aberrant cancer cells and elicits an anti-microtubule response resulting in internalization. T-DM1 is recommended as the second-line treatment in patients with ERBB2-positive metastatic breast cancer who previously received treatment with trastuzumab and taxane (19). And breast cancer patients with ERBB2 protein and mRNA overexpression achieved a better response to T-DM1 (20-23).
However, existing studies concerning NSCLC patients with ERBB2 aberrations have resulted in different conclusions. A basket trial showed an ORR to T-DM1 of 44% in patients with ERBB2 gene mutations (24) and of 43% in patients with ERBB2-amplified lung cancer (25), whereas Hotta and colleagues reported ORRs for ERBB2 gene mutations and amplification as low as 14% and 0%, respectively (26). Another recent study showed that only 4 of 49 patients with ERBB2 protein overexpression showed a partial response (27). This discrepancy could be partly explained by the small sample sizes and single-arm and single-center designs of most studies. In addition, whether concomitance of different ERBB2 alternations affects T-DM1 efficacy is unknown. In the present study, we determined the therapeutic efficacy and safety of T-DM1 on diverse subsets of patients with ERBB2-aberrant NSCLC, by integrating and reanalyzing the data from currently available reports. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2759).
Methods
Search strategy and study selection
We performed a systematic search to select original articles and meeting abstracts in English in PubMed and EMBASE published through June 2020, using combinations of the following key words: (“Trastuzumab emtansine” or “T-DM1”) and (“lung cancer”) and (“HER2” or “HER-2” or “ERBB2”). We reviewed the reference lists of all primary studies, meeting abstracts, and review articles for additional references. All eligible studies were retrieved. Case reports were excluded. When duplicate publications of the same trial were found, the most complete and recent study was included. The criteria for inclusion were studies involving that patients diagnosed with NSCLC harboring ERBB2 aberrations who were treated with T-DM1 regardless of previous treatments, and studies reporting ORRs.
ERBB2 aberrations were defined as ERBB2 gene mutation, gene amplification, or protein overexpression. In our analysis, gene mutations were detected directly by next-generation sequencing (NGS) or polymerase chain reaction (PCR). ERBB2 gene amplification was assessed by fluorescent in situ hybridization (FISH) and defined by an ERBB2/CEP17 ratio of ≥2.0. ERBB2 protein overexpression was assessed by IHC and defined by an IHC score of 2+.
Data extraction and data analysis
Two independent investigators searched and reviewed the articles to exclude irrelevant and overlapping studies, with disagreements resolved by consensus, to ensure the completeness and quality of the analysis. Statistical analysis was carried out in R software (version 3.6.1). The rate is converted and combined using a double inverse sine conversion method. Heterogeneity was assessed using Q test. When P<0.1, the studies were considered to be heterogeneous and a random effects model was adopted, otherwise the fixed effect model was adopted. Publication bias via the Egger test and sensitivity analysis were also estimated.
Results
Study population
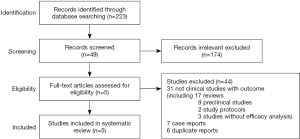
We initially identified 223 studies by full-text screening using the above-mentioned search strategy. However, 174 studies not relevant to our analyses were excluded. After reviewing the full texts of the remaining 49 studies, we excluded 31 studies that were not clinical studies with outcome, including 17 review articles, 9 preclinical studies, 2 study protocols and 3 studies without efficacy analysis. And 7 case reports and 6 duplicate reports were also excluded. Finally, 5 eligible studies involving a total of 120 patients were included in the analysis (Figure 1) (24-28). Of note, 18 patients in study by Li in 2020 (28) were duplicates to those in Li’s study in 2018 (24), therefore only the remaining 31 patients in Li’s study 2020 were involved in analysis.
Among the 120 patients (mean age 62.76 years), ERBB2 aberrations, especially ERBB2 upregulation (protein overexpression and/or gene amplification), were found in current or former smokers (69/113, 61%) with adenocarcinoma (99/113, 88%). There was no significant difference between the sexes (male 50/113, 44% vs. female 63/113, 56%) (Table 1).
Table 1
| Study | Number of patients | Median age [range], year | Female, n [%] | Current and former smoker, n [%] | Adenocarcinoma, n [%] | Stage IV/recurrence, n [%] | Median follow-up time, months | ORR, % [n/N, 95% CI] | Median PFS, month [95% CI] |
|---|---|---|---|---|---|---|---|---|---|
| Hotta 2018 (26) | 15 | 67 [45–77] | 8 [53] | 5 [33] | 15 [100] | 15 [100] | 9.2 | 6.67 [1/15, 0.2–32.0] | 2.0 [1.4–4.0] |
| Li 2018 (24) | 18 | 64 [47–74] | 13 [72] | 11 [61] | 18 [100] | 18 [100] | 10 | 44.44 [8/18, 22–69] | 5 [3–9] |
| Li 2018 (25) | 7 | – | – | – | – | – | – | 42.86 [3/7, 10–82] | 7 [3–13] |
| Peters 2019 (27) | 49 | 61 [36–80] | 20 [41] | 39 [80] | 37 [76] | 49 [100] | 23.1 in IHC 2+ group | 8.16 [4/49, 3–20] | 2.6 [1.4–2.8] in IHC 2+ group; |
| 18.4 in IHC 3+ group | 2.7 [1.4–8.3] in IHC 3+ group | ||||||||
| Li 2020 (28) | 31 | – | 22 [71] | 14 [45] | 29 [94] | 31 [100] | – | 54.84 [17/31, 36–73] | – |
| Total | 120 | 62.76 (mean age) | 63 [56] | 69 [61] | 99 [88] | 113 [100] | – | 21 [4–45] | 3.4 [mean PFS] |
ORR, overall response rate; PFS, progression-free survival; IHC, immunohistochemistry.
Therapeutic efficacy of T-DM1
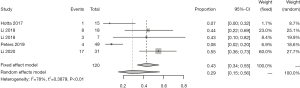
We further analyzed the therapeutic efficacy of T-DM1 on ERBB2 aberrations (Table 2 and Figure 2). The ORR of ERBB2 aberrations in response to T-DM1 was 29% [95% confidence interval (CI): 15–56%]. The ORR was 41% (95% CI: 11–70%) for ERBB2 mutations, 66% (95% CI: 11–100%) for ERBB2 gene amplification, and 3% (95% CI: 0–9%) for ERBB2 protein overexpression. Notably, the ORR for ERBB2 gene mutation was significantly higher than that for ERBB2 upregulation [41% (95% CI: 11–70%) vs. 21% (95% CI: 0–42%)]. The ORR for ERBB2 gene amplification was dramatically greater than that for ERBB2 protein overexpression (66% vs. 3%). These results suggest that T-DM1 is effective for ERBB2-mutated or -amplified lung cancer but has limited efficacy for lung cancer with ERBB2 protein overexpression alone.
Table 2
| Subgroup | ORR, % (95% CI) |
|---|---|
| Aberration type | |
| M | 40.78 (11.15–70.41) |
| O | 2.99 (0.00–8.98) |
| A | 66.29 (11.20–100.00) |
| MO | 26.51 (0.00–54.08) |
| MA | 33.33 (4.33–77.72) |
| AO | 29.01 (0.00–72.30) |
| MAO | 80.28 (50.42–100.00) |
| O+A+AO | 20.80 (0.00–41.89) |
| MO+MA+MAO | 43.87 (24.51–63.24) |
| Total | 28.93 (14.89–56.22) |
| Mutant exon | |
| Exon 20 | 39.52 (18.13–60.91) |
| Exon 19 | 50.00 (0.00–1.00) |
| Exon 17 | 50.00 |
| Exon 8 | 18.62 (0.00–58.49) |
| HER2 rearrangement | 100.00 |
| HER2 SHC-1 fusion | 0 |
PR, partially response; CR, complete response; ORR, overall response rate; M, mutation; O, overexpression; A, amplification; MO, mutation plus overexpression; MA, mutation plus amplification; AO, amplification plus overexpression; MAO, mutation plus amplification plus overexpression.
To investigate the interactions among ERBB2 mutations, amplification, and protein overexpression, we conducted subgroup analyses according to different combinations of ERBB2 aberrations (Table 2). ERBB2 gene mutations combined with upregulation (amplification and/or overexpression) was associated with an ORR of 44% (95% CI: 25–63%), which was identical to that for ERBB2 gene mutations alone (44% vs. 41%). Patients with ERBB2 protein overexpression combined with mutations showed a lower ORR to that for ERBB2 mutations alone [27% (95% CI: 0–54%) vs. 41% (95% CI: 11–70%)], suggesting that ERBB2 overexpression may have a negative interaction effect on ERBB2 mutations in terms of the responsiveness to T-DM1. Notably, patients with triple ERBB2 aberrations showed a higher ORR than that for ERBB2 mutations alone or ERBB2 overexpression combined with mutations [80% (95% CI: 50–100%) vs. 41% (95% CI: 11–70%) vs. 27% (95% CI: 0–54%), respectively]. Collectively, these data suggest the crosstalk between ERBB2 mutations and amplification, eventually resulting in improved responsiveness to T-DM1.
In addition, the effect of various ERBB2 mutations on the efficacy of T-DM1 was assessed (Table 2). ERBB2 exon 20 insertion was the most commonly reported mutation, associated with an ORR of 40% (95% CI: 18–61%). More specifically, the ORR for exon 20 p. A775_G776insYVMA was 43% (95% CI: 22–65%) (Figure 3). However, no meaningful conclusions regarding which mutation was associated with the best T-DM1 efficacy could be reached due to the small sample size.

Safety of T-DM1
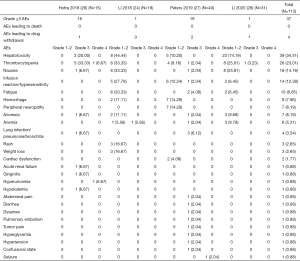
All adverse events (AEs) available for analysis are listed in Figure 4. Among 120 patients from three studies, there are primarily hepatotoxicity (n=39, 35%), thrombocytopenia (n=26, 23%), nausea (n=16, 14%), infusion reaction/hypersensitivity (n=14, 12%), fatigue (n=10, 9%), hemorrhage (n=9, 8%), peripheral neuropathy (n=7, 6%). Totally 37 events (33%) with grade ≥3 AEs are reported. Four patients experienced drug withdrawal due to AEs, one for grade 2 interstitial pneumonia, one for grade 2 infusion reaction, one for grade 3 influenza and one for grade 3 febrile neutropenia. No treatment-related death occurred.
Publication bias and sensitivity analysis
The Egger test showed no publication bias in ORR of ERBB2 aberrations (P=0.08958) in response to T-DM1. The sensitivity analyses demonstrated that almost all estimated values were between the lower and upper confident interval limits (Tables 3,4), hence, the analysis was considered to be stable and the included studies reliable.
Table 3
Table 4
Discussion
The role of ERBB2 as a target of lung-cancer-biomarker-based precision therapies remains poorly understood. In the present analysis, we focused on patients with ERBB2 aberrations treated with T-DM1. ERBB2 aberrations, especially ERBB2 upregulation, tended to occur in current or former smokers with adenocarcinoma, with no significant difference between the sexes. Notably, ERBB2 mutations tended to occur in female nonsmokers with adenocarcinoma. These observations suggest that ERBB2 upregulation, including ERBB2 protein overexpression and gene amplification, is distinctly distributed compared with ERBB2 mutations in NSCLC patients. We further determined whether the specific distribution resulted in diverse responses to T-DM1. By reanalyzing the available data, we concluded that T-DM1 may be an effective agent for lung cancer harboring ERBB2 gene mutations or amplification alone, with limited efficacy for lung cancer harboring ERBB2 protein overexpression alone. However, the question concerning low response rate of T-DM1 in NSCLC harboring ERBB2 overexpression remains unresolved. Study regarding ERBB2 mutation and T-DM1 also found ERBB2 protein expression was low to undetectable levels among ERBB2 mutants and responders (24). Overexpression of ERBB3 protein may serve as an alternative approach of trastuzumab binding and internalization of the antibody-drug conjugate other than through ERBB2 protein overexpression (29). Furthermore, there was no additional effect in cancers harboring ERBB2 protein overexpression combined with other ERBB2 aberrations. However, crosstalk between ERBB2 gene mutations and amplification may have accounted for the additional response to T-DM1 in cancers harboring both of these ERBB2 aberrations.
It has been well-documented that ERBB2 overexpression or gene amplification confers sensitivity to ERBB2-targeting drugs such as trastuzumab, lapatinib, pertuzumab, and T-DM1 in breast cancer patients. T-DM1 was approved for adjuvant treatment of patients with ERBB2-positive early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment (30). Even in patients with locally advanced or metastatic breast cancer treated previously with trastuzumab, T-DM1 showed increased survival benefits in those with ERBB2 amplification and overexpression (19). With the exception of breast cancer, ERBB2 overexpression and gene amplification are rare in lung cancer. Moreover, early studies evaluated trastuzumab monotherapy and trastuzumab in combination with various chemotherapeutic agents, including cisplatin, gemcitabine, docetaxel, and paclitaxel, in patients with ERBB2-positive NSCLC (16,31-35). A lack of trastuzumab activity was consistently observed regardless of the ERBB2 expression status (determined by IHC). The success of trastuzumab in patients with ERBB2-positive breast cancer could not be duplicated in lung cancer patients. However, our findings provide clear evidence confirming that ERBB2 protein overexpression (IHC score of 2+/3+) alone, without gene amplification (FISH negative), did not confer sensitivity to T-DM1 in NSCLC patients. T-DM1 failed to exhibit additional benefits for cancers with concomitant ERBB2 overexpression and mutation compared with those with ERBB2 mutation alone. Although the study from Peters reported a relatively high ORR (20%) for lung cancer patients with ERBB2 overexpression (IHC score of 3+) treated with T-DM1, further evaluation showed that 80% of these patients were also positive for ERBB2 gene amplification by FISH (27). The concordance between FISH and IHC results in lung cancer patients requires extensive validation. In contrast to ERBB2 overexpression, patients with ERBB2 amplification or mutation may benefit from T-DM1 treatment, with a 40–65% ORR and a progression-free survival of approximately 5 months. Notably, the effect of T-DM1 is likely to be synergistic in cases with concomitant ERBB2 amplification and mutation. These data unequivocally support a central role for ERBB2 gene amplification and mutation, but not protein overexpression, as critical biomarkers in predicting the response to T-DM1 in NSCLC patients. Considering the limited value of ERBB2 IHC in NSCLC patients, genomic-based approaches such as NGS should be used routinely to identify ERBB2 gene amplification and mutations. However, the prognoses determined by these laboratory assays require validation in a larger cohort.
ERBB2 gene mutations may be more relevant in lung cancer carcinogenesis compared with ERBB2 upregulation, and several tyrosine kinase inhibitors (TKIs), targeting both EGFR and ERBB2 have been assessed for the treatment of lung cancer harboring ERBB2 aberrations. Afatinib, an EGFR/ERBB1, ERBB2, and ERBB4 inhibitor, was evaluated in our meta-analysis recently. We reported the pooled ORR of 21% and the pooled DCR of 66% in treating ERBB2 mutations. The patients harboring A775-G776ins YVMA mutation, the most common subtype of HER2 exon 20 mutation, derived greater clinical benefit (36). Dacomitinib, another pan-ERBB TKI, was investigated in patients with ERBB2-mutated and ERBB2-amplified lung cancer, with an ORR of 12% (3/26) for ERBB2-mutated cancers but of 0% (0/4) for ERBB2-amplified cancers (37). Neratinib, a TKI targeting EGFR and ERBB2 receptors, showed an ORR of 3.8% (1/26) in patients with ERBB2 mutations (38). Studies of lapatinib, an inhibitor of EGFR and ERBB2, failed to elicit a positive response in patients with ERBB2 gene amplification or mutations. None of five patients with ERBB2 gene mutations achieved a partial response (15), and one of two patients with ERBB2 amplification had unconfirmed tumor shrinkage (39). On the other hand, antibody and antibody drug conjugates also confer sensitivity to HER2 mutations. Trastuzumab and pertuzumab combination therapy was investigated in patients with ERBB2 overexpression plus amplification and mutation. The ORR was 12.5% (2/16) and 21.4% (3/14), respectively. Although limited sample size, mutant patients might benefit more from antibody therapy (40). Trastuzumab deruxtecan (DS-8201a) is an antibody-drug conjugate comprised of an ERBB2-targeting antibody and a topoisomerase I inhibitor. The observed ORR was 61.9% (26/42) in ERBB2-mutated group with median PFS of 14 months (41). Collectively, DS8201a might be a candidate agent targeting ERBB2 aberration, and afatinib showed some degree of efficacy for treatment of ERBB2-mutated lung cancer, whereas neratinib, dacomtinib and lapatinib had very limited therapeutic benefit for ERBB2-aberrant lung cancer.
Our study had some limitations. Because of the low frequency of ERBB2 aberrations, only five studies involving 120 patients were included in our analysis. However, we incorporated all currently available data evaluating the role of T-DM1 in ERBB2-aberrant NSCLC. Secondly, most enrolled studies were single-arm designs with incomplete demographic data which led to the significant heterogeneity between studies, so the evaluation of confounding factors was not definitive. In addition, we only compared ORRs, rather than progression-free survival or overall survival rates, because long-time survival outcomes were not available in the enrolled articles.
The demographics of patients with ERBB2 gene mutations were different from those of patients with gene amplification and protein overexpression. T-DM1 might be an effective and novel agent for lung cancer harboring ERBB2 gene mutations or amplification, with potentially more pronounced therapeutic efficacy for cancers with ERBB2 mutations combined with amplification. However, further clinical trials of T-DM1 treatment of ERBB2-aberrant lung cancer are necessary to confirm our conclusions.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2759
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2759). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099-105. [Crossref] [PubMed]
- Peters S, Zimmermann S. Targeted therapy in NSCLC driven by HER2 insertions. Transl Lung Cancer Res 2014;3:84-8. [PubMed]
- Wang SE, Narasanna A, Perez-Torres M, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10:25-38. [Crossref] [PubMed]
- Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525-6. [Crossref] [PubMed]
- Ninomiya K, Hata T, Yoshioka H, et al. A Prospective Cohort Study to Define the Clinical Features and Outcome of Lung Cancers Harboring HER2 Aberration in Japan (HER2-CS STUDY). Chest 2019;156:357-66. [Crossref] [PubMed]
- Hirsch FR, Varella-Garcia M, Franklin WA, et al. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br J Cancer 2002;86:1449-56. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Heinmöller P, Gross C, Beyser K, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 2003;9:5238-43. [PubMed]
- Kern JA, Slebos RJ, Top B, et al. C-erbB-2 expression and codon 12 K-ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J Clin Invest 1994;93:516-20. [Crossref] [PubMed]
- Harpole DH Jr, Marks JR, Richards WG, et al. Localized adenocarcinoma of the lung: oncogene expression of erbB-2 and p53 in 150 patients. Clin Cancer Res 1995;1:659-64. [PubMed]
- Kinoshita I, Goda T, Watanabe K, et al. A phase II study of trastuzumab monotherapy in pretreated patients with non-small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303-B trial. Ann Oncol 2018;29 Suppl 8:viii540.
- Mazières J, Peters S, Lepage B, et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. Journal of Clinical Oncology 2013;31:1997-2003. [Crossref] [PubMed]
- Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27:281-6. [Crossref] [PubMed]
- Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004;15:19-27. [Crossref] [PubMed]
- de Langen AJ, Jebbink M, Hashemi SMS, et al. Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment. Br J Cancer 2018;119:558-64. [Crossref] [PubMed]
- Zhao J, Xia Y. Targeting HER2 Alterations in Non–Small-Cell Lung Cancer: A Comprehensive Review. JCO Precision Oncology 2020;411-25. [Crossref]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42. [Crossref] [PubMed]
- Yu Q, Zhu Z, Liu Y, et al. Efficacy and Safety of HER2-Targeted Agents for Breast Cancer with HER2-Overexpression: A Network Meta-Analysis. PLoS One 2015;10:e0127404. [Crossref] [PubMed]
- Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011;29:398-405. [Crossref] [PubMed]
- Krop IE, LoRusso P, Miller KD, et al. A Phase II Study of Trastuzumab Emtansine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Who Were Previously Treated With Trastuzumab, Lapatinib, an Anthracycline, a Taxane, and Capecitabine. J Clin Oncol 2012;30:3234-41. [Crossref] [PubMed]
- Perez EA, Hurvitz SA, Amler LC, et al. Relationship between HER2 expression and efficacy with first-line trastuzumab emtansine compared with trastuzumab plus docetaxel in TDM4450g: a randomized phase II study of patients with previously untreated HER2-positive metastatic breast cancer. Breast Cancer Res 2014;16:R50. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 2018;36:2532-7. [Crossref] [PubMed]
- Li BT, Makker V, Buonocore DJ, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36:2502. [Crossref]
- Hotta K, Aoe K, Kozuki T, et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:273-9. [Crossref] [PubMed]
- Peters S, Stahel R, Bubendorf L, et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin Cancer Res 2019;25:64-72. [Crossref] [PubMed]
- Li BT, Michelini F, Misale S, et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov 2020;10:674-87. [Crossref] [PubMed]
- Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009;28:803-14. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Clamon G, Herndon J, Kern J, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670-5. [Crossref] [PubMed]
- Langer CJ, Stephenson P, Thor A, et al. Trastuzumab in the treatment of advanced non-small-cell lung cancer: Is there a role? Focus on eastern cooperative oncology group study 2598. J Clin Oncol 2004;22:1180-7. [Crossref] [PubMed]
- Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218-24. [Crossref] [PubMed]
- Krug LM, Miller VA, Patel J, et al. Randomized phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced nonsmall cell lung carcinoma. Cancer 2005;104:2149-55. [Crossref] [PubMed]
- Zinner RG, Glisson BS, Fossella FV, et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 2004;44:99-110. [Crossref] [PubMed]
- Zhou N, Zhao J, Huang X, et al. The efficacy of afatinib in patients with HER2 mutant non-small cell lung cancer: a meta-analysis. Transl Cancer Res 2020;9:3634-42. [Crossref]
- Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2 - mutant or amplified tumors. Ann Oncol 2015;26:1421-7. [Crossref] [PubMed]
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2-and HER3-mutant cancers. Nature 2018;554:189-94. [Crossref] [PubMed]
- Ross HJ, Blumenschein GR, Aisner J, et al. Randomized Phase II Multicenter Trial of Two Schedules of Lapatinib as First- or Second-Line Monotherapy in Patients with Advanced or Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2010;16:1938-49. [Crossref] [PubMed]
- Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol 2018;36:536-42. [Crossref] [PubMed]
- Smit EF, Nakagawa K, Nagasaka M, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J Clin Oncol 2020;38:9504. [Crossref]