Effects of ANCR lncRNA on the biological behaviors of lung cancer cells A549 and the mechanism
Introduction
Malignant tumors have seriously threatened human health, especially in developing countries. According to the World Health Organization, there are 14 million new cancer cases worldwide annually, and the number of cancer deaths soars in the same period. Particularly, lung cancer has become one of the most common malignant tumors worldwide (1). Therefore, early diagnosis and targeted treatment of lung cancer have attracted widespread attention.
Due to limited means of screening, most patients with lung cancer have already been in the advanced stage upon diagnosis. Therefore, finding effective biomarkers for screening and early diagnosis is of great significance. With in-depth study on the pathogenesis of lung cancer, long non-coding RNA (lncRNA) has been highlighted (2,3). Non-coding RNAs are a class of RNAs with a length of over 200 nucleotides, and lncRNA plays important roles in many life activities such as dose compensation, as well as regulation of epigenetics, cell cycle and differentiation. ANCR lncRNA, which is located on human chromosome 4q12, has been reported to participate in many cancers such as rectal cancer, liver cancer and breast cancer (4-6), but its role in lung cancer remains unclear. FOXO1, as a transcription factor, is involved in the growth inhibition and prognosis of lung cancer (7). ANCR lncRNA can specifically bind FOXO1 and regulate osteoblast differentiation (8). In this study, the expressions of ANCR lncRNA and FOXO1 in lung cancer and adjacent tissues of patients undergoing surgeries in our hospital were detected, and the effects of ANCR lncRNA on the biological behaviors of lung cancer cells, aiming to provide a new target for diagnosis and treatment.
Methods
Materials and reagents
The lung cancer and paracancerous (distance: ≥5 cm) tissues of 50 patients receiving surgeries in our hospital from January 2016 to December 2017 were collected. All cases were pathologically diagnosed as primary lung cancer, without complication with other primary tumors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of our hospital (approval No. ZUCM-FAH-201601003), and written informed consents were obtained from all patients.
Human lung cancer A549 cells (ATCC Cat# CCL-185, RRID: CVCL_0023) were purchased from Cell Bank, Shanghai Institutes for Biological Sciences (China). Lentiviral plasmids packaging ANCR lncRNA and FOXO1 (pHRi-ANCR lncRNA, pHRi-FOXO1), and short hairpin RNA (shRNA) lentiviral expression plasmids (pHRi-sh-ANCR lncRNA, pHRi-sh-FOXO1) were bought from Shanghai GenePharma Co., Ltd. (China). DMEM and fetal bovine serum (FBS) were obtained from Gibco (USA). Reverse transcription kit was provided by Sigma (USA). Lipofectamine 3000 transfection reagent was purchased from Vector (USA). Trizol kit was bought from TaKaRa (Japan). ANCR lncRNA fragment was obtained from Sangon Biotech (Shanghai) Co., Ltd. (China). T7 RNA polymerase was provided by Promega (USA). Biotin RNA Labeling Mix was purchased from Roche (Germany). RNase-free DNase I was bought from Millipore (USA). 5-Ethynyl-2'-deoxyuridine (EdU) kit for cell proliferation detection was obtained from Guangzhou RiboBio Co., Ltd. (China). Apoptosis and cell cycle detection kits were provided by Beyotime Institute of Biotechnology Co., Ltd. (Shanghai, China). Western blot kit was supplied by BD (USA). Rabbit polyclonal antibody anti-FOXO1 (Abcam Cat# ab39670, RRID: AB_732421), as well as rabbit monoclonal antibodies anti-Bax (Abcam Cat# ab32503, RRID: AB_725631), anti-Bcl-2 (Abcam Cat# ab32124, RRID: AB_725644), anti-P27 (Abcam Cat# ab32034, RRID: AB_2244732) and anti-cyclin D1 (Abcam Cat# ab16663, RRID: AB_443423) were purchased from Abcam (USA). Goat anti-rabbit IgG secondary antibody (Signalway Cat# L3011, RRID: AB_895489) was bought from Beijing Zhongshan Jinqiao Biological Technology Co., Ltd. (China). Western blotting was performed by the chemiluminescence method.
Detection of ANCR lncRNA and FOXO1 mRNA expressions in lung cancer tissue by RT-qPCR
ANCR lncRNA: FP: 5'-GCCACTATGTAGAGGGTTTC-3', RP: 5'-ACCTGCGCTAAGAA-CTGAGG-3'; FOXO1: FP: 5'-GGCTGAGGGTTAGTGAGCAG-3', RP: 5'-AAAGGGAGTTGGTGAAAGACA-3'; β-actin: FP: 5'-GCCCATCTATGAGGGTTACGC-3', RP: 5'-GCTTTAGCCACGCTCGGTC-3'. Total RNA was extracted by the Trizol method for the preparation of reverse transcription reaction system and reverse transcription PCR by Go Script™ Reverse Transcription System (Promega, USA). Reaction system: 2 µL of total RNA, 1 µL of primers and 7 µL of RNase-free H2O. The reaction solution was mixed, centrifuged at low speed, incubated at 70 °C for 5 min and immediately cooled on ice. Afterwards, the solution was added 4 µL of 5× GoScriptTM reaction buffer, 1 µL of PCR nucleotide mix, 1 µL of GoScriptTM reverse transcription buffer, 0.5 µL of ribonuclease inhibitor, 1.5 µL of RNase-free H2O and 2 µL of MgCl2. Reaction conditions: Reaction at 25 °C for 5 min, 42 °C for 60 min and 70 °C for 15 min. The obtained cDNA was diluted twice to 5-fold and subjected to real-time PCR with LightCycler 480 PCR System (Roche, Switzerland). PCR system: 10 µL of 2× Taq DNA mix, 1 µL of PCR forward primer (10 µM), 1 µL of PCR reverse primer (10 µM) and cDNA solution. PCR conditions: Pre-denaturation at 75 °C for 2 min, followed by 40 cycles of denaturation at 90 °C for 5 min, annealing at 60 °C for 60 s and extension at 72 °C for 30 s. Relative expression was represented as 2-∆∆Cq. Each sample was tested three times independently.
Cell culture and transfection
A549 cells were cultured in DMEM containing 10% FBS, and the medium was refreshed every two days. The cells were passaged when the confluence reached about 90%. A549 cells in the logarithmic growth phase were transfected with Lipofectamine 3000 according to the manufacturer's instructions.
The cells were divided into control group (untransfected), NC group (transfected with empty lentiviral plasmid), ANCR lncRNA group (transfected with pHRi-ANCR lncRNA), sh-ANCR lncRNA group (transfected with pHRi-sh-ANCR lncRNA), FOXO1 group (transfected with pHRi-FOXO1) and sh-ANCR lncRNA + sh-FOXO1 group (transfected with pHRi-sh-ANCR lncRNA and pHRi-sh-FOXO1).
RNA pull-down assay
RNA pull-down assay was performed according to the kit’s instructions. Total protein was extracted, and a part of the protein lysate was collected as the input control. ANCR lncRNA was labeled with T7 RNA polymerase, and the unlabeled one was used as the negative control. ANCR lncRNA was mixed with streptavidin magnetic beads, left overnight at 4 °C, and placed on ice in cell lysate with labeled RNA for 1 h. RNA-protein binding mixtures were detected by Western blot.
RNA immunoprecipitation (RIP) assay
RIP assay was conducted according to the kit’s instructions. When A549 cells were cultured to 90% confluence, cell suspension was prepared and added RIP buffer. FOXO1 antibody and cell suspension were incubated overnight at 4 °C. Protease K was added for digestion. The supernatant containing RNA was collected and extracted with Trizol. RNA precipitate was analyzed by RT-qPCR. Isotype IgG was used as the negative control, and input was employed as the total protein control.
Detection of cell proliferation by EdU assay
The cells were digested with trypsin, inoculated in 96-well plates at a density of 5×103/well, cultured for 24 h, stained and rinsed according to the kit’s instructions. The cells were thereafter paragraphed under an inverted fluorescence microscope.
Detection of cell cycle and apoptosis by flow cytometry
The cells were digested with trypsin, washed twice with pre-cooled PBS, resuspended with binding buffer and gently blown into a single cell suspension at the concentration of about 1×105. For cell cycle detection, each group was added 5 µL of PI, mixed and left still for 15 min before flow cytometry. For cell apoptosis detection, each group was added 5 µL of annexin V-FITC, mixed, then added 5 µL of 7-AAD and left still at room temperature for 15 min before flow cytometry.
Detection of expressions of FOXO1 and proteins related to cell cycle and apoptosis
Total protein was extracted from tissues and cells with a total protein extraction kit, and the concentration was measured by a BCA protein quantification kit. The protein samples were resolved by 10% SDS-PAGE, and the products were transferred to a PVDF membrane. Subsequently, the membrane was blocked with blocking buffer containing 5% BSA at room temperature for 2 h, incubated with primary antibodies against FOXO1 (1:500), Bax (1:800), P27 (1:1000), Bcl-2 (1:800), cyclin D1 (1:500) and GAPDH (1:1000) overnight at 4 °C, washed three times with buffer, incubated with secondary antibody at room temperature for 1 h, and finally exposed and developed with color development solution. Images were obtained by an automatic gel imaging system, and protein expression levels were measured by using GAPDH as the internal reference.
Statistical analysis
All data were statistically analyzed by SPSS 19.0 software (RRID: SCR_002865), and images were plotted with Graphpad Prism 5.01 software (RRID: SCR_002798). Intergroup comparisons were performed by the t test, and Pearson’s correlation analysis was conducted. P<0.05 was considered statistically significant.
Results
ANCR lncRNA and FOXO1 mRNA expressions in lung cancer and paracancerous tissues and their correlation
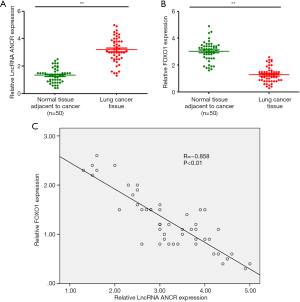
RT-qPCR showed that compared with paracancerous tissue, the ANCR lncRNA expression in lung cancer tissue significantly increased (Figure 1A), whereas that of FOXO1 mRNA significantly decreased (P<0.01) (Figure 1B). Pearson’s correlation analysis revealed that their expressions were negatively correlated (R=−0.858; P<0.01) (Figure 1C).
Correlations between ANCR lncRNA expression and clinicopathological features of lung cancer patients
The relative expressions of ANCR lncRNA in the lung cancer tissues of patients with different gender, age, tumor diameter and pathological type were similar (P>0.05), but such expressions of patients with different TNM stages, differentiation degree and lymph node metastasis had significant differences (P<0.05). In other words, the patients with higher TNM stage, lower differentiation degree and lymph node metastasis had higher relative expression of ANCR lncRNA (Table 1).
Table 1
| Clinicopathological feature | n | ANCR lncRNA expression | P |
|---|---|---|---|
| Gender | 0.293 | ||
| Male | 30 | 3.16±0.98 | |
| Female | 20 | 3.21±0.71 | |
| Age (year) | |||
| <60 | 28 | 3.18±0.87 | |
| ≥60 | 22 | 3.20±0.96 | |
| Tumor diameter (cm) | 0.327 | ||
| <3 | 15 | 3.15±0.92 | |
| ≥3 | 35 | 3.24±0.85 | |
| Pathological type | 0.765 | ||
| Adenocarcinoma | 40 | 3.18±0.94 | |
| Squamous carcinoma | 10 | 3.24±0.79 | |
| TNM stage | 0.000 | ||
| I | 8 | 2.16±0.71 | |
| II | 18 | 3.01±0.99 | |
| III | 16 | 3.51±0.71 | |
| IV | 8 | 4.24±0.29 | |
| Differentiation degree | 0.000 | ||
| Low | 28 | 4.35±0.92 | |
| Moderate | 15 | 3.11±0.89 | |
| High | 7 | 2.14±0.76 | |
| Lymph node metastasis | 0.000 | ||
| Yes | 37 | 4.02±0.92 | |
| No | 13 | 2.96±0.43 |
LncRNA, long non-coding RNA.
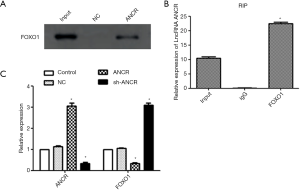
ANCR lncRNA bound FOXO1 and inhibited its expression
RNA pull-down assay showed that there was no FOXO1 protein expression in the NC group, whereas FOXO1 protein was significantly expressed in the ANCR lncRNA group (Figure 2A), indicating that FOXO1 protein was able to bind ANCR lncRNA. RIP assay exhibited that compared with the IgG group, ANCR lncRNA was significantly expressed in the FOXO1 group (Figure 2B), suggesting that ANCR lncRNA can bind FOXO1 protein. The protein expression level of FOXO1, which significantly decreased after overexpression of ANCR lncRNA (P<0.05), significantly increased after knock-down of ANCR lncRNA (P<0.05) (Figure 2C). Collectively, ANCR lncRNA bound FOXO1 protein and suppressed its expression.
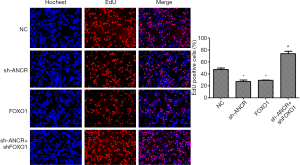
Effects of ANCR lncRNA and FOXO1 on A549 cell proliferation
EdU assay showed that compared with the NC group, the percentage of EdU-positive cells in sh-ANCR lncRNA and FOXO1 groups significantly reduced (P<0.05). Compared with the sh-ANCR lncRNA group, the percentage of the sh-ANCR lncRNA + sh-FOXO1 group was significantly higher (P<0.05) (Figure 3). Therefore, knockdown of ANCR lncRNA obviously inhibited the proliferative ability of A549 cells, which was reversed by inhibiting FOXO1 protein.
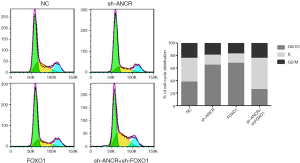
Effects of ANCR lncRNA and FOXO1 on A549 cell cycle
Compared with the NC group, the number of cells in the G0/G1 phase increased significantly in sh-ANCR lncRNA and FOXO1 groups, and the number of cells in the S phase decreased significantly. Compared with the sh-ANCR lncRNA group, the number of cells in the G0/G1 phase of the sh-ANCR lncRNA + sh-FOXO1 group significantly reduced, and the number of cells in the S phase significantly increased (Figure 4). Thus, after knocking down ANCR lncRNA, the A549 cell cycle was mainly arrested in G0/G1 phase, which was reversed through further knockdown of FOXO1 protein.
Effects of ANCR lncRNA and FOXO1 on A549 cell apoptosis
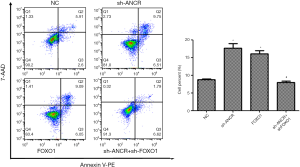
Compared with the NC group, the number of apoptotic cells in sh-ANCR lncRNA and FOXO1 groups significantly increased (P<0.05). Compared with the sh-ANCR lncRNA group, there were significantly fewer apoptotic cells in the sh-ANCR lncRNA + sh-FOXO1 group (P<0.05) (Figure 5). Accordingly, knocking down ANCR lncRNA obviously promoted the apoptosis of A549 cells, which was reversed by further suppressing FOXO1 protein.
ANCR lncRNA regulated A549 cell apoptosis- and cycle-related protein expressions through FOXO1 protein
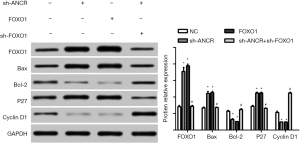
Western blot exhibited that compared with the NC group, the protein expressions of Bax and P27 increased in sh-ANCR lncRNA and FOXO1 groups, but those of Bcl-2 and cyclin D1 decreased significantly (P<0.05). Compared with the sh-ANCR lncRNA group, the expressions of Bax and P27 reduced, whereas those of Bcl-2 and cyclin D1 increased in the sh-ANCR lncRNA + sh-FOXO1 group (P<0.05) (Figure 6). Hence, knockdown of ANCR lncRNA facilitated the expressions of FOXO1 protein, Bax and P27, but inhibited those of Bcl-2 and cyclin D1, thereby suppressing cell cycle progression and promoting apoptosis.
Discussion
The occurrence and development of lung cancer are influenced by many factors. LncRNA, as a genetic molecule involved in cancer biology, has attracted wide attention in recent years. ANCR lncRNA can play a role in promoting or inhibiting cancer in different tumors. For example, Yang et al. found that down-regulating the expression of ANCR lncRNA can inhibit the invasion and metastasis of rectal cancer cells by regulating the expression of EZH2 (4). Li et al. found that ANCR lncRNA was lowly expressed in breast cancer tissues. Overexpression of ANCR lncRNA can inhibit the invasion and metastasis of breast cancer by promoting the ubiquitination and degradation of EZH2 (6). The role of ANCR lncRNA in lung cancer remains unclear. Therefore, in this study, the expression of ANCR lncRNA in lung cancer patients was detected by RT-qPCR, and it was found that the expression of ANCR lncRNA was increased in lung cancer tissues compared with adjacent tissues. Meanwhile, the patients with higher TNM stage, lower differentiation degree and lymph node metastasis had higher relative expression of ANCR lncRNA. Therefore, ANCR lncRNA may play a promotive role in lung cancer.
Tumorigenesis is often accompanied by changes in cell proliferation and growth mode (9). To verify the biological behavior of ANCR lncRNA, in this study, it was knocked down in lung A549 adenocarcinoma cell line, and we found that knockdown of ANCR lncRNA can reduce the proliferation of A549 cells. Furthermore, the number of cells in G0/G1 phase was increased and the number of apoptotic cells was increased. Thus, the inhibition of the ANCR lncRNA expression can inhibit the proliferation of cancer cells, arrest the cancer cell cycle in the G0/G1 phase, and promote the apoptosis of cancer cells.
FOXO1 is an important tumor-negative regulatory transcription factor, which is widely expressed in various human tissues such as lung, heart, and liver (10). It can participate in cell cycle arrest, apoptosis, DNA damage repair by regulating the expression of target genes, inhibit the growth of tumor cells and play an important role in the occurrence, development and prognosis of tumors. Low-level FOXO1 can promote the proliferation of non-small cell lung cancer cells, and FOXO1 has an important blocking effect on the cycle process of lung cancer cells, and induces apoptosis (11,12). Tang et al. found that ANCR lncRNA interacted with FOXO1 and regulated the expression of FOXO1 to inhibit osteoblast differentiation (8).
In order to investigate the role of FOXO1 in lung cancer, this study analyzed whether ANCR lncRNA can play a role in lung cancer by regulating the expression of FOXO1. RT-qPCR showed that FOXO1 was under-expressed in lung cancer tissues. Pearson’s correlation analysis showed that FOXO1 and ANCR lncRNA expressions were negatively correlated in lung cancer tissues. Moreover, RNA pull-down and RIP assays showed that ANCR lncRNA and FOXO1 were bound. Overexpression of ANCR lncRNA can inhibit the expression of FOXO1 expression, while inhibition of ANCR lncRNA can promote the expression of FOXO1. Accordingly, ANCR lncRNA can bind to and inhibit FOXO1 expression in lung cancer A549 cells. Through the measurement of cell proliferation, cycle, and apoptosis, it was further found that overexpression of FOXO1 can inhibit cell proliferation, arrest cells in the G0/G1 phase, and promote apoptosis. Inhibition of FOXO1 on the basis of inhibition of ANCR lncRNA can reverse the inhibition of cell proliferation, cycle arrest and increase of apoptosis, suggesting that inhibition of ANCR lncRNA can regulate cell proliferation, cell cycle and apoptosis by promoting FOXO1 expression, and may inhibit the progression of lung cancer.
P27 protein is a negative regulator of cell cycle, cyclin D1 is a key protein of G1 cell proliferation signal, P27 and cyclin complex can inhibit its activity, prevent the cell cycle from G1 phase to S phase, and thus inhibit cell proliferation (13). Bax and Bcl-2 are both important proteins of apoptosis pathway, which have a greater impact on tumor cell apoptosis (14). To further explore the regulatory mechanism of ANCR lncRNA on lung cancer, this study detected the expression of cycle-related proteins P27, cyclin D1 and apoptosis-related proteins Bax and Bcl-2 in A549 cells after different treatments. Suppression of ANCR lncRNA and overexpression of FOXO1 could promote the expression of P27 and Bax, and inhibit the expression of cyclin D1 and Bcl-2. Compared with the sh-ANCR lncRNA group, the expression of P27 and Bax decreased, and the expression of cyclin D1 and Bcl-2 increased in the sh-ANCR lncRNA + sh-FOXO1 group. Hence, inhibiting ANCR lncRNA can promote the expression of transcription factor FOXO1, activate the expressions of P27 and Bax, and inhibit those of cyclin D1 and Bcl-2, thereby blocking the cell cycle of lung cancer, inhibiting its proliferation and promoting apoptosis. Zhang et al. also showed that inhibiting the expression of ANCR lncRNA in osteosarcoma can promote the expression of P27 (15). Sun et al. found that FOXO1 can be inhibited by miR-27a in liver cancer, leading to a decrease in P27 expression and an increase in cyclin D1 expression, the promotion of the cell cycle transition in G1/S phase, and the induction of proliferation of liver cancer cells (16).
Conclusions
In conclusion, ANCR lncRNA can regulate the proliferation and apoptosis of lung cancer by regulating the expression of FOXO1 in lung cancer, thereby affecting the progression of the disease. ANCR lncRNA is expected to be a potential target for clinical diagnosis of lung cancer, but further in vitro studies using other lung cancer cells and in vivo tests are needed to learn about its role in lung cancer in an in-depth way.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-483
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-483). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of our hospital (approval No. ZUCM-FAH-201601003), and written informed consents were obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu C, Yang Z, Deng Z, et al. Upregulated lncRNA ADAMTS9-AS2 suppresses progression of lung cancer through inhibition of miR-223-3p and promotion of TGFBR3. Iubmb Life 2018;70:536-46. [Crossref] [PubMed]
- Yang R, Li P, Zhang G, et al. Long non-coding RNA XLOC_008466 functions as an oncogene in human non-small cell lung cancer by targeting miR-874. Cell Physiol Biochem 2017;42:126-36. [Crossref] [PubMed]
- Jiang C, Yang Y, Yang Y, et al. Long noncoding RNA (lncRNA) HOTAIR affects tumorigenesis and metastasis of non-small cell lung cancer by upregulating miR-613. Oncol Res 2018;26:725-34. [Crossref] [PubMed]
- Yang ZY, Yang F, Zhang YL, et al. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark 2017;18:95-104. [Crossref] [PubMed]
- Yuan S, Wang J, Yang F, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 2016;63:499-511. [Crossref] [PubMed]
- Li Z, Hou P, Fan D, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ 2017;24:59-71. [Crossref] [PubMed]
- Ding X, Wang Q, Tong L, et al. Long non-coding RNA FOXO1 inhibits lung cancer cell growth through down-regulating PI3K/AKT signaling pathway. Iran J Basic Med Sci 2019;22:491-8. [PubMed]
- Tang Z, Gong Z, Sun X. LncRNA DANCR involved osteolysis after total hip arthroplasty by regulating FOXO1 expression to inhibit osteoblast differentiation. J Biomed Sci 2018;25:4-12. [Crossref] [PubMed]
- Fu T, Coulter S, Yoshihara E, et al. FXR regulates intestinal cancer stem cell proliferation. Cell 2019;176:1098-112.e18. [Crossref] [PubMed]
- Haflidadóttir BS, Larne O, Martin M, et al. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One 2013;8:e72400. [Crossref] [PubMed]
- Zhou Z, Chen G, Deng C, et al. TCF19 contributes to cell proliferation ofnon-small cell lung cancer through inhibiting FOXO1. Cell Biol Int 2019;43:1416-24. [Crossref]
- Gao Z, Liu R, Ye N, et al. FOXO1 inhibits tumor cell migration via regulating cell surface morphology in non-small cell lung Cancer cells. Cell Physiol Biochem 2018;48:138-48. [Crossref] [PubMed]
- Temiz P, Akkaş G, Neşe N, et al. Determination of apoptosis and cell cycle modulators (p16, p21, p27, p53, BCL-2, Bax, BCL-xL, and cyclin D1) in thyroid follicular carcinoma, follicular adenoma, and adenomatous nodules via a tissue microarray method. Turk J Med Sci 2015;45:865-71. [Crossref] [PubMed]
- Zhou S, Wang Y, Zhu JJ. Simultaneous detection of tumor cell apoptosis regulators Bcl-2 and Bax through a dual-signal-marked electrochemical immunosensor. ACS Appl Mater Interfaces 2016;8:7674-82. [Crossref] [PubMed]
- Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res 2017;12:103-9. [Crossref] [PubMed]
- Sun B, Li J, Shao D, et al. Adipose tissue-secreted miR-27a promotes liver cancer by targeting FOXO1 in obese individuals. Onco Targets Ther 2015;8:735-44. [Crossref] [PubMed]