
High expression of carcinoembryonic antigen-associated cell adhesion molecule 1 is associated with microangiogenesis in esophageal squamous cell carcinoma
Introduction
Esophageal cancer, one of the leading causes of cancer death in the world, is a complex disease with sophisticated etiology in human digestive system (1). The two major histological sub-types of esophageal cancer are esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) and the latter is predominant. ESCC is the 4th leading cause of cancer-related death in China (2), which is among the countries with a high incidence of esophageal cancer. It is even the leading cause of death in Taihang Mountain area of North-Central China, where ESCC occurs at the highest rate. Therefore, there is an urgent need to better understand the mechanism of esophageal cancer development and progression, and provide new options for early diagnosis, treatment, and prognosis.
Carcinoembryonic antigen-associated cell adhesion molecule 1 (CEACAM1) is a member of the immunoglobulin superfamily and carcinoembryonic antigen (CEA) family. In human beings, the CEACAM1 gene is about 1.48 kb and encodes 12 different splice variants. CEACAM1 is expressed by certain epithelial, endothelial, lymphoid, and myeloid cells. Previous literatures have been implicated in the functions of CEACAM1 in cell adhesion, immune response, insulin action and angiogenesis. In recent years, there is an increasing number of studies showing that CEACAM1 plays an important role in tumorigenesis by regulating tumor progression, invasion, metastasis, and tumor angiogenesis (3). However, the correlation between esophageal cancer angiogenesis and CEACAM1 is remains unknown.
In this study, we evaluated the protein expression of CEACAM1 and MVD, and then explored the correlation of angiogenesis and CEACAM1 in ESCC.
We present the following article in accordance with the REMARK Reporting Checklist (available at http://dx.doi.org/10.21037/tcr-19-2039).
Methods
Patients
This study was approved by the Ethics Committee of Zhangjiagang Hospital of Traditional Chinese Medicine affiliated to Nanjing University of Chinese Medicine, Suzhou, China (No. 2018-S1072) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). We also received the informed consent of patients. Between January 2010 and January 2016, 80 previously untreated patients of histologically proved ESCC with no distant metastasis were eligible for our study. Cancer stage was recorded based on the International Union Against Cancer (UICC) TNM classification. The median age of the patients at the time of surgery was 63 years, ranging from 50 to 75 years. Fresh primary ESCC tissue samples were derived from patients undergoing surgical procedures. All patients underwent radical esophageal cancer surgery. The clinical features of the patients are listed in Table 1. After completion of primary treatment, patients were followed up every 3 months in the first 2 years, every 6 months in 2–5 years and every year after 5 years. Follow-up evaluation included physical examination, chest radiography, gastroscopy, blood examination and computed tomography scan of chest and abdomen. Within the 40-month median follow-up, the local recurrence, distant metastasis of ESCC and the survival status of patients were verified by clinical records and direct telecommunication with patient or their family.
Table 1
| Variables | No. of patients | MVDa | CEACAM1 expressionb | |||||
|---|---|---|---|---|---|---|---|---|
| High MVD (%) | Low MVD (%) | P-valuec | High expression (%) | Low expression (%) | P-valuec | |||
| Total | 80 | 35 (43.8) | 45 (56.2) | 35 (43.8) | 45 (56.2) | |||
| Tumor staging | ||||||||
| pT1–pT2 | 58 | 23 (39.7) | 35 (60.3) | 0.23 | 24 (41.4) | 34 (58.6) | 0.49 | |
| pT3–pT4 | 22 | 12 (54.5) | 10 (45.5) | 11 (50.0) | 11 (50.0) | |||
| Lymph node involvement | ||||||||
| pN0 | 56 | 22 (39.3) | 34 (60.7) | 0.22 | 17 (30.4) | 39 (69.6) | 0.01 | |
| pN+ | 24 | 13 (54.2) | 11 (45.8) | 18 (75.0) | 6 (25.0) | |||
| Tumor grading | ||||||||
| G1 | 49 | 17 (34.7) | 32 (65.3) | 0.04 | 18 (36.7) | 31 (63.3) | 0.11 | |
| G2–G3 | 31 | 18 (58.1) | 13 (41.9) | 17 (54.8) | 14 (45.2) | |||
| Sex | ||||||||
| Male | 53 | 25 (47.2) | 28 (52.8) | 0.39 | 22 (37.7) | 31 (62.3) | 0.57 | |
| Female | 27 | 10 (37.0) | 17 (63.0) | 13 (55.6) | 14 (44.4) | |||
| Age | ||||||||
| ≤63 years | 42 | 20 (47.6) | 22 (52.4) | 0.46 | 18 (42.9) | 24 (57.1) | 0.86 | |
| >63 years | 38 | 15 (39.5) | 23 (60.5) | 17 (44.7) | 21 (55.3) | |||
a, MVD was categorized according to the median MVD of all tumors into low (< 30 microvessels/400× microscopic field of view) and high (≥30 microvessels/400× microscopic field of view); b, CEACAM1 expression was categorized into low (<66% of CEACAM1 positive tumor cells) and high (≥66% of CEACAM1 positive tumor cells); c, two-sided P-values were calculated by Pearson’s Chi-square test or Fisher’s exact test to evaluate significance of correlations.
Immunohistochemistry (IHC)
CD-105 is considered as a marker of angiogenesis in solid tumors, therefore we used CD105 to evaluate the microvessel density (MVD) (4). Immunohistochemical staining was performed according to standard protocols on formalin fixed paraffin-embedded (FFPE) sections of biopsy samples of ESCC and controls. Slides were incubated with anti-CD105 SN6 (abcam, London, UK) and anti-CEACAM1 (Abnova Biotech Co, Taiwan, China) primary antibody. Then the slides were incubated with Rabbit Anti-Mouse IgG H&L (Biotin) (ab97044) (abcam, London, UK). Antibody binding was visualized using aminoethyl carbazole (AEC) (Biolab, Beijing, China). Immunohistochemical staining was evaluated independently by two pathologists blind to the clinical data.
The MVD in tissues was determined following the previous study of Weidner (5). In brief, the most vascularized areas (hot-spots) within tumor were located under low-power optical magnification (×25) and then microvessels were counted in at least three random microscope fields (×400 magnification) and the average of these fields was recorded. The MVD in tumor samples was classified as low levels (<30 microvessels) and high levels (≥30 microvessels) at ×400 magnification respectively.
The expression of CEACAM-1 was assessed in the same field of MVD. The expression of CEACAM1 in tumor samples was classified as low (<66% positive tumor cells) or high expression (≥66% positive tumor cells) (6) respectively.
Statistical analyses
Statistical analysis was performed using SPSS 22.0 software. All variables were statistically analyzed using crosstab and chi-square test or Fisher’s exact test. For survival analyses, overall survivals stratified by MVD or CEACAM1 expression were presented as the Kaplan–Meier plots and were compared for significance using log-rank testing. Factors such as MVD level, CEACAM1 expression level, pT (pathologic tumor) status, pN (pathologic node) status, tumor histological grade, patient age and gender were analyzed by Cox proportional hazard model to assess the correlation between these variables and MVD or CEACAM1 expression. The P-value lower than 0.05 was considered statistically significant.
Results
Expression analysis of CEACAM1
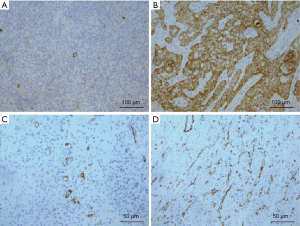
To investigate the clinical significance of CEACAM1 expression in patients with ESCC, we first examined CEACAM1 in human ESCC tissues by IHC. In normal esophageal tissues and stromal cells in ESCC tissues, CEACAM1 was undetectable. Using the criteria described above, high and low expression of CEACAM1 in ESCC was in 35 of 80 (43.8%) (Figure 1A) and 45 of 80 (56.2%) (Figure 1B, Table 1), respectively.
MVD analysis
CD105 was used as a vascular endothelial marker to evaluate MVD in ESCC tissues. The median of microvessels density in ESCC tissues was 30 (ranging from 15 to 55), at ×400 magnification microscope field. Therefore, those ESCC tissue samples were classified as high MVD and low MVD respectively according to the median. High and low MVD was in 35 (43.8%, ≥31 microvessels) and 45 (56.2%, <30 microvessels), respectively, of the ESCCs in the whole cohort at ×400 magnification microscope field (Figure 1C,D, Table 1).
CEACAM1 expression, MVD and correlations with clinicopathologic characteristics
The data analysis showed that MVD and the expression level of CEACAM1 were correlated with lymph node metastasis, depth of tumor invasion, and tumor grade. However, neither MVD nor CEACAM1 was found to be in significant correlation with gender or age (Table 1).
CEACAM1 expression, MVD and their correlations with recurrence, metastasis, and survival
The results of data analysis showed that the expression level of CEACAM1 was positively correlated with the level of MVD (Table 2).
Table 2
| CEACAM1 expression | No. | MVDa | ||
|---|---|---|---|---|
| Low MVD (% per group) | High MVD (% per group) | P-valuec | ||
| Total | 80 | 45 (56.2) | 35 (43.8) | |
| CEACAM1 low expressionb | 45 | 33 (73.3) | 12 (26.7) | 0.001 |
| CEACAM1 high expressionb | 35 | 12 (34.3) | 23 (65.7) | |
a, MVD was categorized according to the median MVD of all tumors into low (< 30 microvessels/400× microscopic field of view) and high (≥30 microvessels/400× microscopic field of view); b, CEACAM1 expression was categorized into low (<66% of CEACAM1 positive tumor cells) and high (≥66% of CEACAM1 positive tumor cells); c, two-sided P-values were calculated by Pearson’s Chi-square test to evaluate significance of correlations.
The median post-intervention follow-up time was 40 months, ranging from 6 to 90 months. Recurrences occurred in 77.5% of patients during the observation period and all these patients suffered cancer-related deaths at the last clinical follow-up. Local recurrence occurred in 20 patients (25%), metastasis in 12 (15%), and synchronous local recurrence and metastasis in 30 (37.5%).
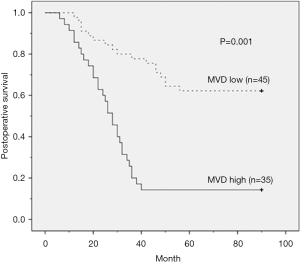
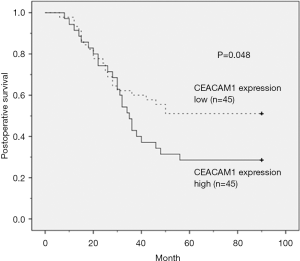
Kaplan-Meier analysis did not show significant correlation between local recurrence and distant metastasis in high MVD and high CEACAM-1 expression groups (Table 3). The Kaplan-Meier survival analysis showed that ESCC patients with high MVD and high CEACAM1 expression had shorter overall survival (P=0.001 and 0.048, respectively; log-rank test, Table 3). These results indicated that high MVD and high CEACAM1 expression level correlate with poor prognosis in ESCC.
Table 3
| MVD and CEACAM1 expression | No. | local recurrence (P-valuec) | Metastasis (P-valuec) | Cancer-related survival (P-valuec) |
|---|---|---|---|---|
| Low MVDa | 45 | 0.089 | 0.112 | 0.001 |
| High MVDa | 35 | |||
| CEACAM1 low expressionb | 45 | 0.563 | 0.21 | 0.048 |
| CEACAM1 high expressionb | 35 |
a, MVD was categorized according to the median MVD of all tumors into low (< 30 microvessels/400× microscopic field of view) and high (≥30 microvessels/400× microscopic field of view); b, CEACAM1 expression was categorized into low (<66% of CEACAM1 positive tumor cells) and high (≥66% of CEACAM1 positive tumor cells); c, P-values of follow-up analyses were determined by log-rank test.
Kaplan-Meier survival curve analysis showed that in high MVD group, 85.7% of the patients died, and in low MVD group 48.9% of the patients died (Figure 2); in high CEACAM1 expression group, 88.5% patients died, while in low CEACAM1 expression group 44.4% of the patients died (Figure 3).
Then we analyzed the correlations of the clinicopathologic factors, such as pT status, pN status, histological grade, age, and gender, with MVD level and CEACAM1 expression level, respectively. The univariate analysis results showed that MVD levels, CEACAM1 expression, lymph node and age could serve as independent prognostic factors for postoperative ESCC. The results of multivariate analysis using Cox proportional hazard regression model indicated that the significance of the prognostic effect of CEACAM-1 expression observed by univariate analysis disappeared when it was analyzed together with MVD, suggesting that the prognostic impact of CEACAM1 expression was dependent on MVD level, while MVD was still a significant prognostic factor for adverse cancer-related survival (P=0.001, Table 4).
Table 4
| Risk factor | Univariate analysis | multivariate analysis | ||
|---|---|---|---|---|
| P-valuea | Relative risk (95% CI) | P-valueb | ||
| MVDe low vs. high | 0.001 | 1.8 (1.0–3.2) | 0.001 | |
| CEACAM1f expression low vs. high | 0.048 | c | 0.362d | |
| pT1–pT2 vs. pT3–pT4 | 0.064 | c | 0.089 | |
| pN0 vs. pN+ | 0.045 | c | 0.115 | |
| G1 vs. G2–G3 | 0.229 | c | 0.325 | |
| male vs. female | 0.354 | c | 0.562 | |
| ≤63 years vs. >63 years | 0.031 | c | 0.052 | |
a, P-values of univariate analyses were determined by log-rank test; b, Stepwise multivariate analysis was performed using the Cox proportional-hazard model; c, no estimate of relative risk is given, since the variable was not significant on multivariate analysis; d, the prognostic impact of CEACAM1 expression is dependent on the prognostic impact of MVD, because when CEACAM1 expression enters multivariate analysis with MVD, the significance of CEACAM1 expression observed by univariate analysis disappears significantly. While MVD itself was still a significant prognostic factor for adverse cancer-related survival; e, MVD was categorized according to the median MVD of all tumors into low (<30 microvessels/400× microscopic field of view) and high (≥30 microvessels/400× microscopic field of view); f, CEACAM1 expression was categorized into low (<66% of CEACAM1 positive tumor cells) and high (≥66% of CEACAM1 positive tumor cells).
Discussion
The 1.48 kb in length CEACAM1 gene, with 9 exons, produces 12 alternatively spliced isoforms of CEACAM1. The seventh exon, 53 bp long, is located at the intracellular end of CEACAM1. The protein sequences encoded by the 7th exon can be removed through selective splicing, generating two transcription products, CEACAM1-L and CEACAM1-S (3).
The roles of CEACAM1 in tumorigenesis and cancer progression are still unclear. CEACAM1 is down-regulated in breast cancer, colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, prostate cancer, and bladder cancer (7-13). However, in pancreatic cancer, gastric cancer, malignant melanoma, thyroid cancer, and lung cancer, CEACAM1 is up-regulated (14-18). CEACAM1 expression is associated with an adverse prognosis in SCC of larynx and tongue (19,20).
To the best of our knowledge, the expression of CEACAM1 in esophageal cancer tissues and its relationship with MVD has not been investigated before. Our results suggest that up-regulation of CEACAM1 is associated with high level of MVD, and CEACAM1 can be a prognostic factor when associated with MVD. High expression of CEACAM1 is correlated with deeper infiltration depth, extensive lymph node metastasis, higher histological grade, advanced tumor stage and poorer survival rate in ESCC.
Therefore, the above data indicate that CEACAM1 might be a useful diagnostic and prognostic molecular marker in ESCC.
What is more, CEACAM1 has a promoting effect on tumor angiogenesis (21). Angiogenesis is a key process in tumorigenesis and progression which is delicately regulated by angiogenic activators and inhibitors (22,23). In this process, cell adhesion molecules play important roles in vascular morphogenesis and endothelial signaling transduction (24). CEACAM1 is one of the adhesion molecules with pro-angiogenic activity. As a major effector of vascular endothelial growth factor (VEGF), CEACAM1 acts as pro-angiogenic factor and supports VEGF-induced endothelial tube formation. Overexpression of CEACAM1 expression induces up-regulation of angiogenic factors such as VEGF, angiopoietin-1 and -2, and this effect can be abolished by application of CEACAM1 antibody, illustrating the pro-angiogenic activity of CEACAM1 (25,26).
The prognostic role of MVD in cancer is still controversial. However, a recent meta-analysis by Ma et al. showed that high MVD correlate with poor prognosis in ESCC, and a study by Wu et al. revealed that MVD was associated with the T-stage of ESCC (27), suggesting that MVD could be a reliable prognostic factor of ESCC, consistent to the result of our study (28).
In our study, the Kaplan-Meier survival analysis showed that high MVD and high CEACAM1 expression were associated with low survival rate. To verify the effect of CEACAM1 expression on the prognosis of ESCC, we used a multivariate Cox regression model to analyze the clinicopathologic characteristics of patients. The results showed that the effect of CEACAM1 on prognosis depended on MVD, because when analyzed together with MVD, the significant effect of CEACAM1 expression observed by univariate analysis disappeared.
CEACAM1 represents a novel multi-purpose therapeutic target for cancer immunotherapy (29,30). CEACAM1 serves as a heterophilic ligand for TIM-3 that is required for its ability to mediate T-cell inhibition. Co-blockade of CEACAM1 and TIM-3 leads to the enhancement of anti-tumour immune responses with improved elimination of tumours in mouse colorectal cancer models (30). Ortenberg R (31) found that the monoclonal antibody (MRG1) could block CEACAM1 function in vivo. CEACAM1 blockade efficiently decreases the growth of melanoma xenografts by induction of specific T-cell–mediated tumor cell apoptosis.
Conclusions
CEACAM1 was reported as a strong clinical predictor of poor prognosis in tumor, since it promoted tumor angiogenesis. Meanwhile, CEACAM1 involves in tumor immune regulation. Identification of CEACAM1 as a prognostic factor in ESCC will provide new insights for diagnosis and prognosis. The results of our study demonstrated a critical role of CEACAM1 in angiogenesis of ESCC progression, thus suggesting that CEACAM1 could be a potential therapeutic target for ESCC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2039
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2039
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2039). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study involving human subjects was approved by the Ethics Committee of Zhangjiagang Hospital of Traditional Chinese Medicine affiliated to Nanjing University of Chinese Medicine (No. 2018-S1072) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients were consent informed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Tang WR, Fang JY, Wu KS, et al. Epidemiological characteristics and prediction of esophageal cancer mortality in China from 1991 to 2012. Asian Pac J Cancer Prev 2014;15:6929-34. [Crossref] [PubMed]
- Kim WM, Huang YH, Gandhi A, et al. CEACAM1 structure and function in immunity and its therapeutic implications. Semin Immunol 2019;42:101296. [Crossref] [PubMed]
- Cho T, Shiozawa E, Urushibara F, et al. The role of microvessel density, lymph node metastasis, and tumor size as prognostic factors of distant metastasis in colorectal cancer. Oncol Lett 2017;13:4327-33. [Crossref] [PubMed]
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995;36:169-80. [Crossref] [PubMed]
- Sienel W, Dango S, Woelfle U, et al. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res 2003;9:2260-6. [PubMed]
- Yang C, He P, Liu Y, et al. Down-regulation of CEACAM1 in breast cancer. Acta Biochim Biophys Sin (Shanghai) 2015;47:788-94. [Crossref] [PubMed]
- Yang C, Cao M, Liu Y, et al. Inhibition of cell invasion and migration by CEACAM1-4S in breast cancer. Oncol Lett 2017;14:4758-66. [Crossref] [PubMed]
- Song JH, Cao Z, Yoon JH, et al. Genetic alterations and expression pattern of CEACAM1 in colorectal adenomas and cancers. Pathol Oncol Res 2011;17:67-74. [Crossref] [PubMed]
- Kiriyama S, Yokoyama S, Ueno M, et al. CEACAM1 long cytoplasmic domain isoform is associated with invasion and recurrence of hepatocellular carcinoma. Ann Surg Oncol 2014;21:S505-14. [Crossref] [PubMed]
- Kammerer R, Riesenberg R, Weiler C, et al. The tumour suppressor gene CEACAM1 is completely but reversibly downregulated in renal cell carcinoma. J Pathol 2004;204:258-67. [Crossref] [PubMed]
- Zhang H, Eisenried A, Zimmermann W, et al. Role of CEACAM1 and CEACAM20 in an in vitro model of prostate morphogenesis. PLoS One 2013;8:e53359. [Crossref] [PubMed]
- Tilki D, Singer BB, Shariat SF, et al. CEACAM1: a novel urinary marker for bladder cancer detection. Eur Urol 2010;57:648-54. [Crossref] [PubMed]
- Gebauer F, Wicklein D, Horst J, et al. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS One 2014;9:e113023. [Crossref] [PubMed]
- Shi JF, Xu SX, He P, et al. Expression of carcinoembryonic antigen-related cell adhesion molecule 1(CEACAM1) and its correlation with angiogenesis in gastric cancer. Pathol Res Pract 2014;210:473-6. [Crossref] [PubMed]
- Ashkenazi S, Ortenberg R, Besser M, et al. SOX9 indirectly regulates CEACAM1 expression and immune resistance in melanoma cells. Oncotarget 2016;7:30166-77. [Crossref] [PubMed]
- Ueshima C, Kataoka TR, Takei Y, et al. CEACAM1 long isoform has opposite effects on the growth of human mastocytosis and medullary thyroid carcinoma cells. Cancer Med 2017;6:845-56. [Crossref] [PubMed]
- Ling Y, Wang J, Wang L, et al. Roles of CEACAM1 in cell communication and signaling of lung cancer and other diseases. Cancer Metastasis Rev 2015;34:347-57. [Crossref] [PubMed]
- Lucarini G, Zizzi A, Re M, et al. Prognostic implication of CEACAM1 expression in squamous cell carcinoma of the larynx: Pilot study. Head Neck 2019;41:1615-21. [Crossref] [PubMed]
- Wang N, Wang Q, Chi J, et al. Carcinoembryonic antigen cell adhesion molecule 1 inhibits the antitumor effect of neutrophils in tongue squamous cell carcinoma. Cancer Science 2019;110:519-29. [Crossref] [PubMed]
- Fiori V, Magnani M, Cianfriglia M. The expression and modulation of CEACAM1 and tumor cell transformation. Ann Ist Super Sanita 2012;48:161-71. [Crossref] [PubMed]
- Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med 2004;255:538-61. [Crossref] [PubMed]
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med 2003;3:643-51. [Crossref] [PubMed]
- Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost 2001;86:308-15. [Crossref] [PubMed]
- Ergün S, Kilik N, Ziegeler G, et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell 2000;5:311-20. [Crossref] [PubMed]
- Kilic N, Oliveira-Ferrer L, Wurmbach JH, et al. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem 2005;280:2361-9. [Crossref] [PubMed]
- Wu HL, Guan BX, Liu B, et al. The intrapapillary capillary loop (IPCL) changes in superficial esophageal lesions. Dis Esophagus 2017;30:1-5. [PubMed]
- Ma G, Zhang J, Jiang H, et al. Microvessel density as a prognostic factor in esophageal squamous cell cancer patients: A meta-analysis. Medicine (Baltimore) 2017;96:e7600. [Crossref] [PubMed]
- Dankner M, Gray-Owen SD, Huang YH, et al. CEACAM1 as a multi-purpose target for cancer immunotherapy. Oncoimmunology 2017;6:e1328336. [PubMed]
- Huang YH, Zhu C, Kondo Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015;517:386-90. [Crossref] [PubMed]
- Ortenberg R, Sapir Y, Raz L, et al. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Mol Cancer Ther 2012;11:1300-10. [Crossref] [PubMed]


