
The incidence and risk factors of acute radiation-induced dermatitis in gynecologic malignancies treated with intensity-modulated radiation therapy
Introduction
Intensity-modulated radiation therapy (IMRT) is widely used due to its advantages. Despite its positive benefits, however, skin damage to superficial tissues or organs after irradiation has drawn increasing attention. Although several studies have confirmed that IMRT causes acute radiation-induced dermatitis (RID), most were limited to head and neck tumours, as well as breast cancer (1-3). Researchers believe that late RID after radiotherapy, which leads to decrustation and fibrosis, may be associated with acute RID. Furthermore, RID caused by IMRT seriously affects quality of life (4-6). However, among gynaecological malignancies, RID caused by radiotherapy has scarcely been reported. When IMRT is applied to treat the region including the lower vagina and/or inguinal lymph nodes, acute RID of varied severity usually occurs (7). However, the incidence of such common complications and treatments regarding acute RID remains unclear. Once patients experience severe acute RID, radiotherapy must be interrupted (8), resulting in prolonged length of hospital stay, which in turn may lead to treatment failure (9,10). Traditionally, treatment of acute RID usually consists of supportive management of its symptoms. The benefits of triethanolamine emulsion for preventing acute RID remain controversial. Several studies have confirmed that triethanolamine emulsion may help to prevent acute RID, while other investigations have not drawn the same conclusion. Therefore, the incidence, risk factors, and most efficient treatment for acute RID is of significant concern in patients with gynaecological malignancies who undergo IMRT.
This retrospective study aimed to analyse the incidence of and risk factors for acute RID in gynaecological malignancies in patients undergoing IMRT, and to explore the role of triethanolamine emulsion in the prevention of acute RID. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-796).
Methods
Study design
Patients diagnosed with gynaecological malignancies, who underwent lower vaginal and/or inguinal IMRT between January 2012 and June 2014 at the Zhejiang Cancer Hospital (Hangzhou, Zhejiang Province, China), were enrolled, including those with cervical cancer with lower-third vagina invasion, vaginal cancer with lower vagina invasion, and vulvar cancer with inguinal node invasion. All malignancies were confirmed by pathology. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). And it was approved by the Zhejiang Cancer Hospital Ethics Committee (Reference number, IRB-2018-91), with informed consent taken from all the patients.
Treatment
IMRT was used for external irradiation of the pelvic cavity, and the entire vagina and groin. The X-ray radiation energy was 10 MV, and the total dose was 45–46.4 Gy, which was applied in fractional doses of 1.6–1.8 Gy. For metastatic inguinal lymph node(s) confirmed by pathology, the nodal dose was boosted to 54–60 Gy. The maximum dose to the skin was limited to 50 Gy. During external irradiation, patients were positioned supine for IMRT with closed legs, and without any tissue compensation. After external irradiation, patients with cervical cancer and/or vaginal cancer were treated with high-dose brachytherapy. The synchronous chemotherapy regimens included the following:
- Cisplatin mono-chemotherapy, with a dose of 40 mg/m2, once per week;
- 5-fluorouracil plus cisplatin (FP) regimen: 5-fluorouracil 1,000 mg/m2·d continuous intravenous pumping for 96 h plus cisplatin 70 mg/m2 intravenous infusion on the first day, every 3 weeks;
- Paclitaxel plus cisplatin (TP) regimen: paclitaxel 135 mg/m2 plus cisplatin 70 mg/m2 intravenous infusion on the first day, every 3 weeks.
Triethanolamine emulsion (Biafine, Laboratoire Medix, Buc, France) was applied to the inguinal and vulvar skin three times per day, starting from the first day of radiotherapy to the conclusion of radiotherapy.
Data collection
Characteristic data, including age, body height and weight, hyperglycaemia, tumour stage and pathological data, were collected. Hyperglycaemia was defined as venous fasting blood glucose level ≥7.1 mmol/L for 2 consecutive measurements. Body mass index (BMI) was calculated based on height and weight. Meanwhile, treatment data were recorded, including dose of radiotherapy, radiation boost, synchronous chemotherapy, and its specific regimen. Moreover, prophylactic use of the triethanolamine emulsion and severity of RID were analysed. The severity of acute RID was classified into 5 grades according to the Radiation Therapy Oncology Group (RTOG) criteria (11). Acute skin toxicity in the inguinal and valvular area were recorded. Of all grades of skin toxicity during the treatment, the most serious was graded.
Statistical analyses
All statistical analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Continuous data are expressed as mean and standard deviation, and the categorical data are expressed as frequency and percentage. The χ2 test was used to compare acute skin toxicity between different groups. A logistic regression model was adopted for multivariate analysis; differences with P<0.05 was considered statistically significant.
Results
Characteristics and outcomes
In the present study, 96 patients, with a median age of 56 years (range, 33 to 84 years) were enrolled. The median BMI was 22.01 kg/m2, with minimum of 16.65 kg/m2 and maximum of 29.64 kg/m2. According to the Chinese criteria for overweight and obese, of 96 patients, 8 had a BMI of <18.5 kg/m2, 68 had a BMI between 18.5 and 24 kg/m2, and the remaining 20 had a BMI of >24 kg/m2 (Table 1).
Table 1
| Characteristics | Grades 0–1 | Grades 2–4 | P value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age, years | 0.521 | |||||
| <40 | 2 | 4.5 | 4 | 7.7 | ||
| 40–50 | 13 | 29.6 | 14 | 26.9 | ||
| 51–60 | 12 | 27.3 | 20 | 38.5 | ||
| >60 | 17 | 38.6 | 14 | 26.9 | ||
| BMI (kg/m2) | 0.893 | |||||
| <18.5 (n) | 4 | 9.1 | 4 | 7.7 | ||
| 18.5–24 (n) | 30 | 68.2 | 38 | 73.1 | ||
| >24 (n) | 10 | 22.7 | 10 | 19.2 | ||
| Comorbidity | ||||||
| Hyperglycemia | 0.033* | |||||
| Yes | 6 | 13.6 | 17 | 32.7 | ||
| No | 38 | 86.4 | 35 | 67.3 | ||
| Treatment | ||||||
| Synchronous chemotherapy | 0.011* | |||||
| Yes | 23 | 52.3 | 40 | 76.9 | ||
| No | 21 | 47.7 | 12 | 23.1 | ||
| Radiation boost | 0.870 | |||||
| Yes | 3 | 6.8 | 4 | 7.7 | ||
| No | 41 | 93.2 | 48 | 92.3 | ||
| Triethanolamine emulsion | 0.015* | |||||
| Yes | 27 | 61.4 | 19 | 36.5 | ||
| No | 17 | 38.6 | 33 | 63.5 | ||
*, P<0.05. BMI, body mass index.
Pathological analysis confirmed 72 cases of cervical cancer, 23 vaginal cancer, and 1 vulvar cancer. According to histopathology, 93 cases were squamous cell carcinoma, 2 were adenocarcinoma and 1 was malignant melanoma; 63 patients underwent synchronous chemotherapy together with radiotherapy, among whom 30 received cisplatin mono-chemotherapy, another 8 underwent the FP regimen, and the remaining 25 underwent the TP regimen.
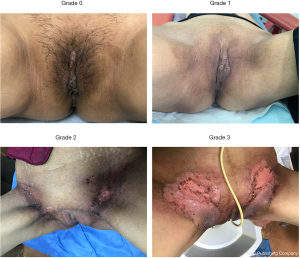
Of all patients, 23 (23.9%) had accompanying hyperglycaemia. Triethanolamine emulsion was prophylactically used in 46 (47.9%) patients; 7 patients were treated with a boost dose for inguinal lymph node. According to the RTOG criteria, acute RID in patients in this study was graded from 0 to 4, as follows: grade 0 (n=2; 2.1%); grade 1 (n= 42; 43.8%); grade 2 (n=34; 35.4%); grade 3 (n=18; 18.8%); grade 4 (n=0; 0%) (Table 2, Figure 1) (12). Acute RID was confirmed in 94 (97.9%) patients, of whom 52 (55.3%) were grade ≥2 severity, which required further clinical intervention.
Table 2
| Grade | Skin | No. of patients (%) |
|---|---|---|
| Grade 0 | No change over baseline | 2/96 (2.1) |
| Grade 1 | Follicular, faint, or dull erythema/epilation/dry desquamation/decreased sweating | 42/96 (43.8) |
| Grade 2 | Tender or bright erythema, patchy moist desquamation/ moderate edema | 34/96 (35.4) |
| Grade 3 | Confluent, moist desquamation other than skin folds, pitting edema | 18/96 (18.8) |
| Grade 4 | Ulceration, hemorrhage, necrosis | 0/96 (0) |
RID, radiation-induced dermatitis; RTOG, radiation therapy oncology group.
Univariate analysis of acute RID for its risk factors
All variables were included in the univariate analysis, including age, BMI, hyperglycaemia, synchronous chemotherapy, radiation boost, and prophylactic use of triethanolamine emulsion. The presence of grade ≥2 severity RID was significantly associated with synchronous chemotherapy (P=0.011), hyperglycaemia (P=0.029), and prophylactic use of triethanolamine emulsion (P=0.015). However, other variables, including age, radiation boost and BMI, failed to reach statistical significance (t=0.482, P=0.631; t=0.027, P=0.870; and t=0.276, P=0.599, respectively) (Table 3).
Table 3
| Parameter | t/χ2 | P value |
|---|---|---|
| Age | 0.482 | 0.631 |
| BMI classification | 0.276 | 0.599 |
| Hyperglycemia | 4.750 | 0.029* |
| Synchronous chemotherapy | 6.420 | 0.011* |
| Radiation boost | 0.027 | 0.870 |
| Prophylactic use of triethanolamine emulsion | 5.886 | 0.015* |
*, P<0.05. BMI, body mass index; RID, radiation-induced dermatitis.
Multivariate analysis of acute RID
Based on the results of univariate analysis, all factors with P<0.1 were entered into the multivariate analysis for association with grade ≥2 severity RID. Three independent factors were included: hyperglycaemia, synchronous chemotherapy, and prophylactic use of triethanolamine emulsion. Because radiation boost has been reported to have significant influence on RID, it was also entered into the logistic multivariate analysis. Grade ≥2 severity RID was independently correlated with synchronous chemotherapy [odds ratio (OR) 3.515; 95% CI, 1.362–9.072; P=0.009], hyperglycaemia (OR 3.150; 95% CI, 1.019–9.736, P=0.046), and prophylactic use of triethanolamine emulsion (OR 0.412; 95% CI, 0.170–0.998; P=0.049). Similar to the univariate analysis, radiation boost demonstrated no statistical significance (P=0.927) (Table 4).
Table 4
| Variable | β | SE | Wald | OR (95% CI) | P |
|---|---|---|---|---|---|
| Synchronous chemotherapy | 1.257 | 0.484 | 6.752 | 3.515 (1.362–9.072) | 0.009** |
| Triethanolamine | −0.886 | 0.451 | 3.861 | 0.412 (0.170–0.998) | 0.049* |
| Radiation boost | −0.077 | 0.841 | 0.008 | 0.925 (0.178–4.811) | 0.927 |
| Hyperglycemia | 1.147 | 0.576 | 3.971 | 3.150 (1.019–9.736) | 0.046* |
*, P<0.05; **, P<0.01. RID, radiation-induced dermatitis.
Discussion
Radiotherapy is of significant importance in the treatment of gynaecological malignancies. IMRT is less toxic to normal tissues, especially the intestine, colon, and rectum (13). However, acute RID of the vulva and inguinal region of individuals undergoing IMRT has been observed in clinical practice (7). Although the mechanism remains unclear, RID raises an urgent concern for its origin. To our knowledge, the present study was the first to focus on its incidence and risk factors in individuals undergoing IMRT for gynaecological malignancies. The present study attempted to fill this information gap in the literature and raise awareness to it.
Although we limited the maximum dose of inguinal and vulvar skin to 50 Gy during IMRT, 35.4% of patients experienced grade 2 acute RID and 18.8% grade 3 acute RID. It has been established that the severity of acute RID directly influences quality of life and interrupts the consistency of radiotherapy, which ultimately negatively affects the expected therapeutic benefit (14,15).
To investigate risk factors for acute RID, for the first time, we used univariate and multivariate analysis for patients with gynaecological malignancies who underwent IMRT. The results indicated that synchronous chemotherapy and hyperglycaemia were independent risk factors for grade ≥2 severity acute RID in the lower vaginal or inguinal region while receiving IMRT. Moreover, the use of triethanolamine emulsion during radiotherapy may help to relieve acute RID.
Acute RID is a common complication in radiotherapy for gynaecological malignancies. Maduro et. al. reported that the incidence of acute RID during radiotherapy in cervical cancer could reach 27% (16). Moreover, synchronous chemotherapy will increase the toxicity of treatments for gynaecological malignancies (17,18). However, to date, the role of synchronous chemotherapy in acute RID has not been described. To our knowledge, this was the first study to confirm synchronous chemotherapy as one independent risk factor for grade ≥2 severity acute RID. This implies that if IMRT was applied to the lower vaginal and inguinal region, synchronous chemotherapy could increase the risk for acute RID and, as such, more attention and precautions are needed in advance. However, the effect of specific chemotherapy regimens on acute RID requires further study.
Results of this study revealed that hyperglycaemia may aggravate acute RID in the vulva or inguinal region during IMRT. The mechanism between hyperglycaemia and acute RID in radiotherapy remains unclear. It may be partially due to damage of the vascular wall of the skin caused by radiotherapy and delayed repair due to hyperglycaemia. Moreover, hyperglycaemia can trigger peripheral microangiopathy and neuropathy, which may further aggravate the RID caused by radiotherapy. A few studies have focused on acute RID and hyperglycaemia. Wazer et al. indicated that diabetes was not significantly correlated with skin toxicity in IMRT for breast cancer (19). However, a history of diabetes, instead of actual blood glucose level when undergoing radiotherapy, was used in a previous study, which may conceal the role of blood glucose level in acute RID. Moreover, the relationship between hyperglycaemia and acute RID during radiotherapy in gynaecological malignancies has not been reported. According to the results of this study, hyperglycaemia was an independent risk factor for acute RID during IMRT in patients with gynaecological malignancies. For patients diagnosed with hyperglycaemia before irradiation, controlling blood glucose to normal levels may help to reduce the incidence of acute RID.
Several studies have indicated that radiation boost in head and neck cancer (HNC) would increase the dose of radiation in the targeted region, ultimately inducing and aggravating acute RID (20). However, in gynaecological malignancies, no such report(s) has been published. Our study demonstrated that radiation boost was not correlated with the severity of RID, both in univariate and multivariate analyses, indicating that radiation boost may not worsen RID. Compared with published studies, the highest radiation dose in HNC was usually >65 Gy (20,21), while in our study the highest radiation dose was boosted to 55–60 Gy, significantly less than that for HNC. This may partially explain the disparity of the role of radiation boost for HNC and gynaecological malignancies.
Use of prophylactic triethanolamine emulsion was reported to help reduce acute RID during radiotherapy in previous research; however, most of the targeted cancers were restricted to HNC and breast cancer (12,22). For gynaecological malignancies, few studies have focused on the efficacy of triethanolamine emulsion during IMRT, although in breast cancer, one study reported no significant advantage(s) of triethanolamine over other management strategies (15). However, Abbas et al. confirmed the superiority of triethanolamine emulsion in reducing the severity of acute dermatitis in HNC (12). Compared with breast cancer and HNC, radiotherapy to targeted regions in the groin and lower vagina is at significantly higher risk for acute RID of the vulva and inguinal skin, because the skin in these areas is more wrinkled. Thus, it is clinically meaningful to explore efficient management strategies to prevent or reduce acute RID. Our research indicated that the prophylactic use of triethanolamine emulsion also helped to decrease clinically relevant acute RID, suggesting that this emulsion may confer a protective effect on the skin in the groin and vulva. Therefore, continuous prophylactic use of triethanolamine emulsion throughout IMRT is suggested, and may provide a benefit for reducing the incidence of acute RID.
There were some limitations to our study. First, all data analysed were collected retrospectively, which may have resulted in bias. Therefore, further prospective randomised controlled trials with more objective evaluation are warranted. Second, only 96 patients were enrolled; as such, the sample size may have been insufficient and an expanded database is needed for further analysis. Third, high individual variation exists in the development of RID; therefore, more variables are needed to be stratified to investigate the “dose-effect” of each responsible determinant. For example, the relationships between the severity of RID and varying blood glucose levels, specific chemotherapy regimen, and drug dose remain to be further investigated, which may finally help to guide individual treatment during IMRT.
Conclusions
In conclusion, IMRT for the lower vagina and groin region in individuals with gynaecological malignancies complicated by considerable acute RID cannot be neglected. Moreover, hyperglycaemia and synchronous chemotherapy were independent predictive factors for clinically relevant acute RID. Prophylactic use of triethanolamine emulsion may help to reduce the occurrence of acute RID.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-796
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-796
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-796). All authors report grants from Zhejiang Provincial Natural Science Foundation of China, grants from Science and Technology Program of Zhejiang Chinese Traditional Medicine, grants from Zhejiang Medical and Health Science and Technology Project, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in this study involving human participants were consistent with the ethical standards of Helsinki declaration (as revised in 2013). The ethical committee of Zhejiang Cancer Hospital approved this study (Reference number IRB-2018-91), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Langhe S, Mulliez T, Veldeman L, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer 2014;14:711. [Crossref] [PubMed]
- Borghini A, Vecoli C, Mercuri A, et al. Genetic risk score and acute skin toxicity after breast radiation therapy. Cancer Biother Radiopharm 2014;29:267-72. [Crossref] [PubMed]
- Duprez F, Berwouts D, Madani I, et al. High-dose reirradiation with intensity-modulated radiotherapy for recurrent head-and-neck cancer: Disease control, survival and toxicity. Radiother Oncol 2014;111:388-92. [Crossref] [PubMed]
- Tanteles GA, Whitworth J, Mills J, et al. Can cutaneous telangiectasiae as late normal-tissue injury predict cardiovascular disease in women receiving radiotherapy for breast cancer? Br J Cancer 2009;101:403-9. [Crossref] [PubMed]
- Lilla C, Ambrosone CB, Kropp S, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007;106:143-50. [Crossref] [PubMed]
- Keller LM, Sopka DM, Li T, et al. Five-year results of whole breast intensity modulated radiation therapy for the treatment of early stage breast cancer: the Fox Chase Cancer Center experience. Int J Radiat Oncol Biol Phys 2012;84:881-7. [Crossref] [PubMed]
- Jeon W, Koh HK, Kim HJ, et al. Salvage radiotherapy for lymph node recurrence after radical surgery in cervical cancer. J Gynecol Oncol 2012;23:168-74. [Crossref] [PubMed]
- Krusun S, Pesee M, Supakalin N, et al. Treatment interruption during concurrent chemoradiotherapy of uterine cervical cancer: analysis of factors and outcomes. Asian Pac J Cancer Prev 2014;15:5653-7. [Crossref] [PubMed]
- Lim A, Sia S. Outcomes of chemoradiotherapy in cervical cancer--the Western Australian experience. Int J Radiat Oncol Biol Phys 2012;82:1431-8. [Crossref] [PubMed]
- Song S, Rudra S, Hasselle MD, et al. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer 2013;119:325-31. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer 2012;20:185-90. [Crossref] [PubMed]
- Vandecasteele K, Tummers P, Makar A, et al. Postoperative intensity-modulated arc therapy for cervical and endometrial cancer: a prospective report on toxicity. Int J Radiat Oncol Biol Phys 2012;84:408-14. [Crossref] [PubMed]
- Salvo N, Barnes E, van Draanen J, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol 2010;17:94-112. [PubMed]
- Geara FB, Eid T, Zouain N, et al. Randomized, Prospective, Open-label Phase III Trial Comparing Mebo Ointment With Biafine Cream for the Management of Acute Dermatitis During Radiotherapy for Breast Cancer. Am J Clin Oncol 2018;41:1257-62. [Crossref] [PubMed]
- Maduro JH, Pras E, Willemse PHB, et al. Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat Rev 2003;29:471-88. [Crossref] [PubMed]
- Roy S, Maji T, Chaudhuri P, et al. Addition of gemcitabine to standard therapy in locally advanced cervical cancer: A randomized comparative study. Indian J Med Paediatr Oncol 2011;32:133-8. [Crossref] [PubMed]
- Hackbarth M, Haas N, Fotopoulou C, et al. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women's cancers. Results of a prospective study. Support Care Cancer 2008;16:267-73. [Crossref] [PubMed]
- Wazer DE, Kaufman S, Cuttino L, et al. Accelerated partial breast irradiation: an analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2006;64:489-95. [Crossref] [PubMed]
- Studer G, Brown M, Salgueiro EB, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys 2011;81:110-7. [Crossref] [PubMed]
- Selzer E, Liederer S, Lemaire C, et al. Incidence of dermatitis in head and neck cancer patients treated with primary radiotherapy and cetuximab. Strahlenther Onkol 2011;187:373-7. [Crossref] [PubMed]
- Elliott EA, Wright JR, Swann RS, et al. Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol 2006;24:2092-7. [Crossref] [PubMed]


