
Expression of CMTM4 shows clinical significance in lung cancer
Introduction
Lung cancer is a malignant tumor that has the highest incidence and mortality around the world, which has become a serious public health problem (1). In China, lung cancer is also one of the most threatening malignant tumors to the health of the population (2). Lung cancer is divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) according to the clinical and histopathological characteristics of patients, and NSCLC accounts for about 80%. NSCLC is further classified into squamous cell carcinoma, adenocarcinoma and large cell carcinoma (3). The most common histologic subtype of lung cancer in most countries is lung adenocarcinoma (4). The etiology of lung cancer is still not completely clear, a large number of data show that long-term heavy smoking has a very close relationship with the occurrence of lung cancer (5). Studies have shown that long-term smokers are 10 to 20 times more likely to develop lung cancer than nonsmokers (6,7). Early diagnosis and treatment of lung cancer is the key to improve the survival rate and prognosis of lung cancer patients.
In recent years, there are many studies focusing on cancer biomarkers, and emerging biomarkers have been shown to play a role in cancer. As a novel gene family, the human chemokine-like factor (CKLF)-like MARVEL transmembrane domain-containing family (CMTM) is implicated in reproduction, immunity, and carcinogenesis. High expression of CMTM2 and CMTM3 was found in the male testes and important in spermatogenesis (8,9). Furthermore, CMTM4 cooperates with its family member CMTM6, can participate in immune escape through synergistic protection of PD-L1 (10). CMTM1 was found to be associated with chemotherapy resistance and prognosis in patients with NSCLC (11). Our previous studies have identified that CMTM2, CMTM4, CMTM6, and CMTM7 were down-regulated in hepatocellular carcinoma (HCC) tissues (12-15). These findings suggest that CMTM family members are potential important cancer biomarkers for cancer diagnosis and therapy.
CMTM4 is the most conserved chemokine containing the MARVEL domain on chromosome 16q22.1 (16). CMTM4 exhibits tumor suppressive roles in renal clear cell carcinoma (CCRCC) and can be an effective target for CCRCC therapy (17), as well as a risk factor of poor prognosis for HCC (13). It was found that CMTM4 could cause cell cycle arrest at G2/M phase and inhibit HeLa cell proliferation without inducing apoptosis (18). Moreover, there was an interaction between CMTM4 and PD-L1 in cancer tissues. CMTM4 could protect PD-L1 as a target for lysosomal degradation and prevent the clearance of tumor cells from body immune cells, showing an important role of CMTM4 in cancer immunotherapy (10). However, the expression and clinical significance of CMTM4 in lung cancer have not been elucidated.
To explore the expression and clinical significance of CMTM4 in lung cancer, we conducted immunohistochemistry (IHC) analysis in 75 paired lung adenocarcinoma and non-tumor lung tissues. We found that CMTM4 was down-regulated in lung cancer tissues and had a relationship with metastasis and prognosis in lung cancer patients.
We present the following article in accordance with the REMARK reporting checklist (available at: http://dx.doi.org/10.21037/tcr-20-1254).
Methods
Tissue samples
Seventy-five paired lung adenocarcinoma and non-tumor lung tissues were collected from the Affiliated Guilin People’s Hospital of Guilin Medical University from 2014 to 2016. These cases were diagnosed as lung cancer by pathological method and had no treatment before operation. All patients had detailed clinical data and signed the informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Guilin Medical University (GLMC2014003).
The clinic-pathological features of lung cancer patients were shown in Table 1. Some variables in Table 1 were defined as follows. Patients had a history of smoking, alcohol intake, allergy, and diseases diagnosed were defined as “Yes” of smoking, alcohol intake, allergy history, and past history. According to the latest WHO classification (19), these cases were all invasive adenocarcinomas and classified into 5 subtypes, acinar predominant, solid predominant, papillary predominant, mucinous adenocarcinoma, and fetal adenocarcinoma. The TNM classification was conducted according to 8th Edition of the TNM Classification for Lung Cancer (20).
Table 1
| Variables | Total | CMTM4 staining | P | |
|---|---|---|---|---|
| Negative | Positive | |||
| Gender | 0.035* | |||
| Male | 43 | 19 | 24 | |
| Female | 32 | 22 | 10 | |
| Age, year | 0.324 | |||
| <50 | 12 | 5 | 7 | |
| ≥50 | 63 | 36 | 27 | |
| Smoking | 0.010* | |||
| No | 43 | 29 | 14 | |
| Yes | 32 | 12 | 20 | |
| Alcohol intake | 1.000 | |||
| No | 67 | 37 | 30 | |
| Yes | 8 | 4 | 4 | |
| Allergy history | 0.498 | |||
| No | 73 | 39 | 34 | |
| Yes | 2 | 2 | 0 | |
| Past history | 0.648 | |||
| No | 57 | 32 | 25 | |
| Yes | 18 | 9 | 9 | |
| Family history of lung cancer | 0.453 | |||
| No | 74 | 41 | 33 | |
| Yes | 1 | 0 | 1 | |
| CEA (ng/mL) | 0.837 | |||
| <5 | 56 | 31 | 25 | |
| ≥5 | 19 | 10 | 9 | |
| CA125 (U/mL) | 1.000 | |||
| <35 | 73 | 40 | 33 | |
| ≥35 | 2 | 1 | 1 | |
| CA199 (U/mL) | 1.000 | |||
| ≤37 | 74 | 40 | 34 | |
| >37 | 1 | 1 | 0 | |
| Pathological classification | 0.251 | |||
| Acinar predominant | 31 | 15 | 16 | |
| Solid predominant | 13 | 7 | 6 | |
| Papillary predominant | 22 | 11 | 11 | |
| Mucinous adenocarcinoma | 5 | 5 | 0 | |
| Fetal adenocarcinoma | 4 | 3 | 1 | |
| Tumor diameter (cm) | 0.311 | |||
| <5 | 55 | 32 | 23 | |
| ≥5 | 20 | 9 | 11 | |
| TNM stage | 1.000 | |||
| I+II | 69 | 38 | 31 | |
| III+IV | 6 | 3 | 3 | |
| Lymphatic metastasis | 0.030* | |||
| No | 36 | 15 | 21 | |
| Yes | 39 | 26 | 13 | |
*, indicate significance.
IHC
The tissues were made on a tissue microarray from Fanpu (Guilin, China). And then the IHC assay was performed to detect the expression of CMTM4 by an IHC kit (Maixin biotechnologies, Fuzhou, China) according to the previous method (14). After incubated with primary CMTM4 antibody (NBP1-84457, Novus, CO, USA) and secondary anti-rabbit antibody, the tissue microarray was color-developed by 3,3’-aminobenzidine tetrahydrochloride (DAB), and observed under a microscope for 5–10 min until the appropriate color appeared. And then it was counterstained with hematoxylin, dehydrated, transparent, and fixed.
The IHC results were read by two professional pathologists according to the intensity of staining and the percentage of positive cells. For the inconsistency of the results, a third pathologist was invited to reread the tissue microarray. Five visual fields (×400) were observed in each section. The score for percentage of positive cells was defined as: 0 for ≤10%, 1 for 11–25%, 2 for 26–50%, 3 for 51–75%, and 4 for >75%. The score for intensity of staining was defined as: 0 for no color, 1 for light color, 2 for middle, and 3 for dark brown color. H-score (percentage × intensity) is for CMTM4 expression. H-score >4 was defined as positive expression of CMTM4 and ≤4 was defined as negative expression of CMTM4.
Statistical analysis
All data were statistically analyzed by SPSS 19.0 software. Chi-square test was used to analyze the expression difference of CMTM4 between lung adenocarcinoma and non-tumor lung tissues. Kaplan-Meier method and Log-Rank test were used to compare the survival curves between different groups. P<0.05 was defined as statistically significant.
Results
CMTM4 is down-regulated in lung cancer tissues
We first analyzed the expression of CMTM4 in lung adenocarcinoma tissues from TCGA database according to the previous method (21). As shown in Figure 1A, there was no difference of CMTM4 expression between lung adenocarcinoma tissues and normal lung tissues in TCGA database (P>0.05). Then the expression of CMTM4 in lung adenocarcinoma and corresponding non-tumor lung tissues was identified by IHC staining. As shown in Figure 1B, CMTM4 was mainly located in the cytoplasm and cell membrane. Moreover, CMTM4 was positively expressed in 34/75 (45.3%) cases of lung adenocarcinoma tissues, while positively expressed in 59/75 (78.6%) cases of non-tumor lung tissues (Table 2). After the McNemar χ2 test, the positive expression of CMTM4 in 75 cases of lung adenocarcinoma tissues was significantly lower than that in the paired non-tumor lung tissues (P<0.05). The IHC results suggest that CMTM4 is down-regulated in lung cancer tissues.
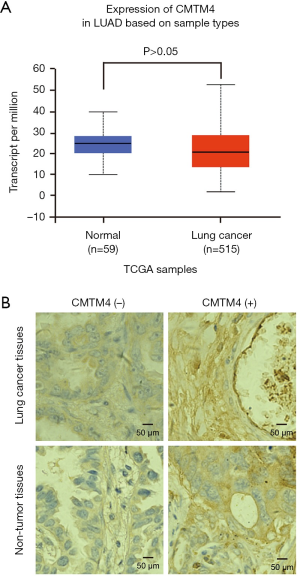
Table 2
| Lung cancer tissues | Adjacent non-tumor tissues | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 30 | 4 | 34 |
| Negative | 29 | 12 | 41 |
| Total | 59 | 16 | 75 |
P<0.05, P value is based on the McNemar χ2 test.
CMTM4 expression is related with the clinic-pathological features of lung cancer patients
According to the IHC results of CMTM4 expression, we divided the 75 cases of lung cancer patients into CMTM4 positive group and CMTM4 negative group. The clinic-pathological features of these patients were collected to analyze the relationship with CMTM4 expression, such as gender, age, smoking, alcohol intake, allergy history, past history, family history, CEA, CA125, CA199, pathological classification, tumor diameter, TNM stage, and metastasis. After chi-square test, we found that CMTM4 expression had a significant correlation with gender, smoking, and metastasis (P<0.05, Table 1), but was not related with other clinic-pathological features of lung cancer patients (P>0.05). In addition, through multivariate logistic regression analysis, we confirmed that CMTM4 expression was only significantly positive correlated to smoking in these lung cancer patients (OR=3.452, P<0.05, Table 3).
Table 3
| Variables | B | Wald | P value | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Smoking | 1.239 | 6.417 | 0.011* | 3.452 | 1.324 | 9.005 |
*, indicate significance.
CMTM4 expression is correlated with the prognosis of lung cancer patients
Furthermore, we performed a survival analysis to explore the relationship between CMTM4 expression and the prognosis of these lung cancer patients. As shown in Figure 2, the expression of CMTM4 was significantly associated with the survival of these lung cancer patients (P=0.012). Moreover, the survival rate of these patients in the CMTM4 negative group was significantly lower than that in the CMTM4 positive group. In addition, we did COX regression analysis on the overall survival of lung cancer patients after surgery and found that CMTM4 expression was an independent factor affecting the prognosis of these lung cancer patients (OR=2.003, 95% CI: 1.152–3.483), P<0.05, Table 4).
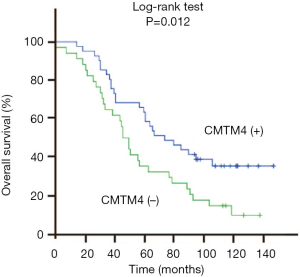
Table 4
| Variables | B | Wald | P value | ORa | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| CMTM4 | 0.695 | 6.067 | 0.014* | 2.003 | 1.152 | 3.483 |
| Smoking | −0.112 | 0.159 | 0.690 | 0.894 | 0.515 | 1.551 |
*, indicate significance. a, adjusted for age and gender.
Discussion
As a leading cause of death in both men and women worldwide, lung cancer has become a global problem and attracted a lot of attention. In this study, we found that the expression of CMTM4 was lower in lung adenocarcinoma tissues than that in the corresponding non-tumor tissues. Moreover, down-regulated CMTM4 was associated with gender, smoking, and metastasis in lung cancer patients. In addition, CMTM4 expression was an independent prognostic factor for the overall survival of lung cancer patients. Our study suggests a suppressor role of CMTM4 in lung cancer.
CMTM family members are widely expressed and play an important role in various cellular processes, especially in carcinogenesis. Increased expression of CMTM1_v17 in breast cancer cells can promote cell proliferation and resistance to tumor necrosis factor-α (TNF-α)-induced apoptosis (22). Apart from affecting sperm and testosterone production, CMTM2 is also found down-regulated in HCC tissues and associated with the prognosis of HCC patients (12). CMTM3 is an independent prognostic marker for gastric cancer (23), while CMTM5 is an independent indicator of prognosis for oral squamous cell carcinoma (24), and CMTM7 is an independent prognostic factor in NSCLC patients (25). CMTM8 regulates cell proliferation and apoptosis by influencing EGFR and related signaling pathways through the MARVEL region (26).
In consistent with its family members, CMTM4 also shows anti-tumorigenic activity in several cancers, such as CCRCC, HCC, and colorectal cancer (13,17,27). Our study detected down-regulated CMTM4 expression in lung adenocarcinoma tissues, further supporting its suppressor role in lung cancer. The reason is mainly in the location of the CMTM4 gene on chromosome 16q22.1, a locus that harbors a number of tumor-suppressor genes (16). Furthermore, in accord with our previous study of CMTM4 in HCC (13), this study also found that the expression of CMTM4 was associated with some clinic-pathological features of lung cancer patients, gender, smoking, and metastasis. As all we know, smoking is the main risk factor for lung cancer. Anti-smoking strategies are the most effective and efficient way to reduce the high incidence and mortality of lung cancer (28). Through the relationship between CMTM4 expression and smoking status of lung cancer patients, we can conclude some etiology of lung cancer caused by smoking. The exact mechanism needs to be further explored in other models. Of particular concern, the identification of CMTM4 as a critical PD-L1 regulator provides that CMTM4 can be used as an immunotherapeutic target for cancer (10). Our study also showed that the negative expression of CMTM4 was correlated with shorter survival time of lung cancer patients than the positive expression of CMTM4, implying a potential prognosis marker of CMTM4 in lung cancer.
In addition, there are some limits in our study. First, we detected CMTM4 expression only in lung adenocarcinoma tissues and the sample size was small. Second, we had small proportion of patients in high stage and didn’t exclude the other possible therapy that patients had received after surgery. Third, we didn’t detect PD-L1 expression to see its correlation with CMTM4. In future, we need to increase the sample size and select more subtype of lung cancer to solve these shortcomings.
Conclusions
In conclusion, we first clarified that CMTM4 was down-regulated in lung adenocarcinoma tissues and correlated to gender, smoking, and metastasis in lung cancer patients. Furthermore, down-regulated CMTM4 expression in lung cancer tissues had a significant relationship with the prognosis of patients. Our findings suggest CMTM4 as a potential biomarker that can be used in lung cancer diagnosis and prognosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the REMERK reporting checklist. available at http://dx.doi.org/10.21037/tcr-20-1254
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1254
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1254). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients had detailed clinical data and signed the informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the medical ethics committee of Guilin Medical University (GLMC2014003).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mao Y, Yang D, He J, et al. Epidemiology of Lung Cancer. Surg Oncol Clin N Am 2016;25:439-45. [Crossref] [PubMed]
- Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019;10:3-7. [Crossref] [PubMed]
- Jia L, Zhang J, Ma T, et al. The Function of Fucosylation in Progression of Lung Cancer. Front Oncol 2018;8:565. [Crossref] [PubMed]
- Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and Molecular Classification of Lung Cancer. Cancer Treat Res 2016;170:25-46. [Crossref] [PubMed]
- Yoshida K, Gowers KHC, Lee-Six H, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 2020;578:266-72. [Crossref] [PubMed]
- Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004;328:1519. [Crossref] [PubMed]
- Boffetta P, Pershagen G, Jockel KH, et al. Cigar and pipe smoking and lung cancer risk: a multicenter study from Europe. J Natl Cancer Inst 1999;91:697-701. [Crossref] [PubMed]
- Zhang XW, Yin HQ, Li Q, et al. CMTM2 is involved in spermiogenesis in mice]. Beijing Da Xue Xue Bao Yi Xue Ban 2019;51:228-93. [PubMed]
- Li Z, Xie J, Wu J, et al. CMTM3 inhibits human testicular cancer cell growth through inducing cell-cycle arrest and apoptosis. PLoS One 2014;9:e88965. [Crossref] [PubMed]
- Mezzadra R, Sun C, Jae LT, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017;549:106-10. [Crossref] [PubMed]
- Si J, Zhang P, Tian D, W, et al. CMTM1_v17 is associated with chemotherapy resistance and poor prognosis in non-small cell lung cancer. World J Surg Oncol 2017;15:34. [Crossref] [PubMed]
- Guo X, Zhang S, Tan S, et al. Downregulated CMTM2 Poses Potential Clinical Significance in Hepatocellular Carcinoma. DNA Cell Biol 2020;39:683-9. [Crossref] [PubMed]
- Bei C, Zhang Y, Wei R, et al. Clinical significance of CMTM4 expression in hepatocellular carcinoma. Onco Targets Ther 2017;10:5439-43. [Crossref] [PubMed]
- Zhu X, Qi G, Li C, et al. Expression and Clinical Significance of CMTM6 in Hepatocellular Carcinoma. DNA Cell Biol 2019;38:193-7. [Crossref] [PubMed]
- Zeng W, Zhu X, Luo W, et al. Clinical significance of expression of CMTM7 in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2016:4568-75.
- Wang Y, Li J, Cui Y, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res 2009;69:5194-201. [Crossref] [PubMed]
- Li T, Cheng Y, Wang P, et al. CMTM4 is frequently downregulated and functions as a tumour suppressor in clear cell renal cell carcinoma. J Exp Clin Cancer Res 2015;34:122. [Crossref] [PubMed]
- Plate M, Li T, Wang Y, et al. Identification and characterization of CMTM4, a novel gene with inhibitory effects on HeLa cell growth through Inducing G2/M phase accumulation. Mol Cells 2010;29:355-61. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017;19:649-58. [Crossref] [PubMed]
- Wang J, Zhang G, Zhang Y, et al. CMTM1_v17 is a novel potential therapeutic target in breast cancer. Oncol Rep 2014;32:1829-36. [Crossref] [PubMed]
- Yuan W, Liu B, Wang X, et al. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett 2017;386:77-86. [Crossref] [PubMed]
- Zhang H, Nan X, Li X, et al. CMTM5 exhibits tumor suppressor activity through promoter methylation in oral squamous cell carcinoma. Biochem Biophys Res Commun 2014;447:304-10. [Crossref] [PubMed]
- Liu B, Su Y, Li T, et al. CMTM7 knockdown increases tumorigenicity of human non-small cell lung cancer cells and EGFR-AKT signaling by reducing Rab5 activation. Oncotarget 2015;6:41092-107. [Crossref] [PubMed]
- Huang YM, Du Y. Effect of novel human chemokine-like factor superfamily 8 on proliferation and EGFR expression of tumor cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006;22:466-8. [PubMed]
- Xue H, Li T, Wang P, et al. CMTM4 inhibits cell proliferation and migration via AKT, ERK1/2, and STAT3 pathway in colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2019;51:915-24. [Crossref] [PubMed]
- Ruano-Ravina A, Provencio-Pulla M, Fernandez-Villar JA. Promotion of Anti-Smoking Strategies as the Most Effective and Efficient Way to Reduce Lung Cancer (and Other Diseases). Arch Bronconeumol 2020;56:339. [PubMed]


