
Treatment outcomes for hepatoblastoma children with pulmonary metastasis and extrapulmonary involvement: experience of 36 cases at a single institution
Introduction
Hepatoblastoma (HB) is the most common primary liver malignant tumor in children, and has a prevalence of 0.9 per million population (1,2). HB usually affects children under 3 years of age, presenting as a large abdominal mass (3). The diagnosis of HB is initially performed based on the elevated alpha-fetoprotein (AFP) level and radiographic detection of a liver mass, and confirmed by pathological examination of samples obtained via either primary liver resection or biopsy (4). Several factors are reported to have a significant impact on prognosis, including PRETEXT stage, pathology type, AFP level at diagnosis and after chemotherapy, and distant metastasis (5-8).
In the 1970s, the main treatment for HB was single tumor resection, which leaded to a very low overall survival (OS) rate (20% to 30%) (9). Since the 1980s, the comprehensive treatment of surgical combined with chemotherapy has significantly improved prognosis, and the 5-year survival rate could reach 75% (10,11). However, patients with advanced HB usually have poor prognosis, especially those with an extensive unifocal or multifocal primary tumor or distant metastases (12). About one fifth of the patients have lung metastasis at diagnosis, and the recurrence of HB mostly occurred in the lung (13,14). In addition, reports on the prognosis of HB with extrapulmonary involvement are relatively rare (2,15,16). Therefore, we aimed to retrospectively analyze the clinical characteristics and outcome in HB children with pulmonary metastasis and extrapulmonary involvement in this study and provide some valuable information for further treatment. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1876).
Methods
Patients
We retrospectively reviewed 36 HB patients with pulmonary metastasis and extrapulmonary involvement from January 2010 to December 2017. The inclusion criteria were as follows: (I) patients diagnosed with pulmonary metastasis and extrapulmonary involvement HB for the first time in our hospital; (II) patients with age <14 years; (III) lung enhanced computed tomographic (CT) or positron emission tomographic (PET)-CT showed that patients with metastatic nodules; (IV) patients with extrapulmonary involvement had corresponding imaging evidence. Patients who received any prior treatments were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Beijing Tongren Hospital, Capital Medical University (No. 20180212) and informed consent was taken from all participants and their guardians.
Diagnostic criteria
The diagnosis was based on the clinical examination, operative biopsy or percutaneous needle biopsy. Histology was mainly classified as epithelial (including embryonal, macrotrabecular and fetal subtypes) or mixed type (17). HB staging (stage I-IV) was performed according to the PRETEXT staging guidelines established by the International Childhood Liver Tumor Strategy Group (SIOPEL) (18). Risk stratification (extremely low risk, low risk, intermediate risk, high risk) for HB was based on both a SIOPEL risk stratification system and a Children’s Oncology Group (COG) staging system (19,20).
Therapy
The study design was formulated with reference to CHIC (15), COG (21-23) and other organizations. Patients received comprehensive treatment. Patients with PRETEXT stage III and IV received pre-surgical chemotherapy, surgery and post-surgical chemotherapy. In addition, patients with PRETEXT stage II underwent complete resection and adjuvant chemotherapy. Pre-surgical chemotherapy was sustained for 3–5 cycles. Post-surgical chemotherapy was sustained for 4–6 cycles. Twenty-one to twenty-eight days were one cycle of chemotherapy. The commonly used chemotherapy regimens were VICC (vincristine+irinotecan+cyclophosphamide+cisplatin) and VIFC (vincristine+irinotecan+fluorouracil+cisplatin) (24). Serum AFP levels, blood routine test, blood biochemical routine and electrocardiogram were detected in each cycle. Primary and metastatic lesions were evaluated every 2 cycles of chemotherapy. The surgeries included hepatectomy, thrombectomy, and lung mass resection. Other treatment measures (interventional therapy, radiofrequency ablation, ultrasound focused scalpel, etc.) were selected according to the tumor condition.
Analysis of therapeutic effect and follow-up
The prognosis of all patients was indicated by OS, which was calculated from the day of first admission to our hospital to the time of the last follow-up or death. Censored cases were defined as patients who died from non-tumor causes. The evaluation criteria of chemotherapy efficacy were partial response (PR) and complete response (CR). PR was defined as a reduction of the product of the largest perpendicular diameters of all measurable lesions by more than 50%. CR was defined as disappearance of all known tumor lesions. Recurrence was defined as biopsy confirmation, with clear imaging evidence and serum AFP increased 3 times continuously within 4 weeks. The patients were followed up to December 2018, with a median follow-up of 32.5 months.
Statistical analysis
All statistical analyses were performed by using SPSS version 21.0 (SPSS Institute, IL, USA). Quantitative data were expressed as means ± standard deviations (SD) and were compared using Student’s t-test. Qualitative data were expressed as number and percentage and were compared using χ2 test. Survival curves were calculated by Kaplan-Meier method. Statistical significance was set at P<0.05.
Results
Demographic and clinical characteristics
A total of 36 HB patients (26 males, 10 females; median age, 2.13 years; range, 0.33–7.83 years) with pulmonary metastasis and extrapulmonary involvement were enrolled in this retrospective study from January 2010 to December 2017. The demographic and clinical characteristics of included patients were shown in Table 1.
Table 1
| Characteristic | Number (n, %) |
|---|---|
| Gender | |
| Male | 26 (72.22) |
| Female | 10 (27.78) |
| Pathology type | |
| Epithelial | 62 (62.27) |
| Mixed | 36 (36.73) |
| Not clearly classified | 1 (2.78) |
| PRETEXT stage | |
| Stage III | 12 (33.33) |
| Stage IV | 24 (66.67) |
| Serum AFP (μg/L) | |
| <100,000 | 21 (58.33) |
| ≥100,000 | 15 (41.67) |
| Pulmonary metastasis | |
| Single nodule in one lung | 4 (11.11) |
| Multiple nodules in both lungs | 32 (88.89) |
| Extrapulmonary involvement | |
| Blood vessels | 16 (44.44) |
| Extrahepatic abdomen | 10 (27.78) |
| Mediastinum | 3 (8.33) |
| Brain | 13 (36.11) |
| Spinal cord | 1 (2.78) |
| Bone | 4 (11.11) |
| Bone marrow | 1 (2.78) |
HB, hepatoblastoma; AFP, alpha-fetoprotein.
Among the 36 patients, 18 (50.00%) were epithelial subtype, 17 (47.22%) were mixed subtype and 1 (2.78%) was not clearly classified. There were 12 (33.33%) patients with PRETEXT stage III and 24 (66.67%) patients with PRETEXT stage IV. The median serum AFP level was 52,000 µg/L (range, 726–25,009,220). Four (11.11%) patients presented with single metastatic pulmonary nodules, 32 (88.89%) patients presented with multiple metastatic nodules in both lungs. There were 10 (27.78%) patients with extrahepatic abdomen metastasis, 3 (8.33%) patients with mediastinum metastasis, 13 (36.11%) patients with brain metastasis (Figure 1), 1 (2.78%) patients with spinal cord metastasis, 4 (11.11%) patients with bone metastasis (Figure 2), 16 (44.44%) patients with vascular metastasis (Figure 3), and 1 (2.78%) patients with bone marrow metastasis.
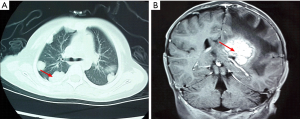
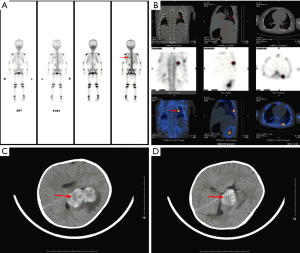
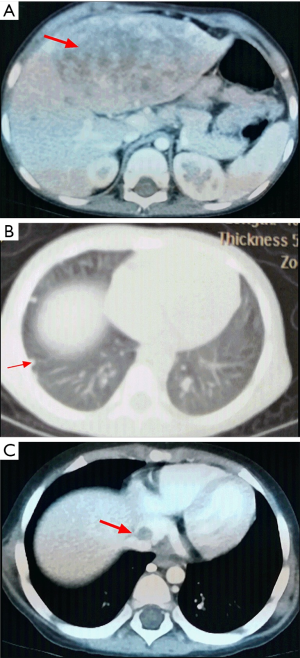
Treatment and outcomes
All patients were treated with surgery and chemotherapy (Table 2). All patients underwent liver tumorectomy, of which 24 (68.57%) patients underwent 1-time liver tumorectomy, 9 (25.71%) patients underwent 2 times liver tumorectomy, and 3 (8.57%) patients underwent 3 times liver tumorectomy. Otherwise, 35 (97.22%) patients received standardized chemotherapy for more than 6 cycles, and 1 (2.78%) patient only received three cycles of chemotherapy. The median chemotherapy cycle was 17 (range, 3–39), of which pre-surgical median chemotherapy cycle was 3 (range, 0–8) and post-surgical median chemotherapy cycle was 14 (range, 0–39). In addition, 19 (54.29%) patients underwent lung metastasectomy, of which 10 (28.57%) patients underwent 1 time lung metastasectomy, 3 (8.57%) patients underwent 2 times lung metastasectomy, 5 (14.29%) patients underwent 3 times lung metastasectomy, and 1 (2.86%) patient underwent 4 times lung metastasectomy.
Table 2
| Characteristics | Number (n, %) |
|---|---|
| Standard treatment | |
| No | 1 (2.78) |
| Yes | 35 (97.22) |
| Liver tumorectomy | 35 (100.00) |
| 1 time | 24 (68.57) |
| 2 times | 9 (25.71) |
| 3 times | 3 (8.57) |
| Lung metastasectomy | 19 (54.29) |
| 1 time | 10 (28.57) |
| 2 times | 3 (8.57) |
| 3 times | 5 (14.29) |
| 4 times | 1 (2.86) |
| Surgery on other organs | |
| Inferior vena cava tumor thrombectomy | 3 (8.57) |
| Right atrial tumor thrombectomy | 2 (5.71) |
| Portal vein tumor thrombectomy | 1 (2.86) |
| Mediastinal tumorectomy | 2 (5.71) |
| Intracranial tumorectomy | 3 (8.57) |
| Spinal and adrenal tumorectomy | 1 (2.86) |
| Recurrence and progression | |
| Liver recurrence | 14 (40.00) |
| Lung recurrence | 18 (51.42) |
| Head recurrence | 11 (31.43) |
| Mediastinal recurrence | 1 (2.86) |
| Progression after liver tumorectomy | 3 (8.57) |
| Prognosis | |
| CR | 7 (20.00) |
| PR | 2 (5.71) |
| Dead | 24 (68.57) |
| Censored | 3 (8.57) |
CR, complete remission; PR, partial remission.
Patients were followed up to December 2018, with a median follow-up of 32.5 months. At the study closing date, 9 patients were alive, 24 patients had died, and 3 patients were censored (died from non-tumor causes). In addition, 14 (40.00%) patients had liver recurrence, 18 (51.42%) patients had lung recurrence, 11 (31.43%) patients had head recurrence, and 1 (2.86%) patient had mediastinal recurrence.
Survival analysis
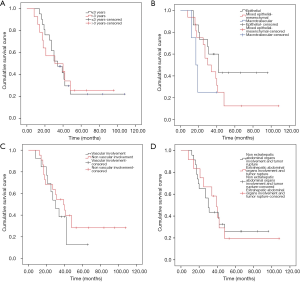
The survival curve of all patients was shown in Figure 4A. The median OS was 32.5 months (range, 8–108). For 35 patients receiving standardized chemotherapy, the survival time was 47.16±6.33 months, the 3-year OS rate was 48.6%, and the 5-year OS rate was 23.7%. For 1 patient who only received three cycles of chemotherapy after surgery, the survival time was 17 months.
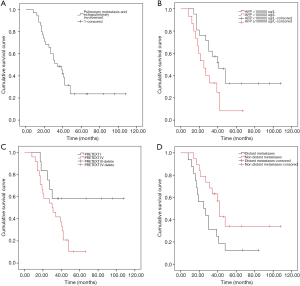
Considering the level of serum AFP (Figure 4B), the OS of patients with serum AFP <100,000 µg/L was longer than that of patients with AFP ≥100,000 µg/L (56.21±8.68 vs. 28.57±4.19 months, χ2=4.511, P=0.034). PRETEXT stage III was associated with a longer disease specific survival (OS, 72.75±12.08 months) compared with PRETEXT stage IV (OS, 32.04±3.44 months) (χ2=4.148, P=0.042) (Figure 4C). In addition, the OS for patients with distant metastases (brain and bone metastasis) and without distant metastases were 32.00±5.60 and 58.05±9.34 months, respectively (χ2=4.620, P=0.032) (Figure 4D).
No statistically significant differences in OS were identified when the study group was divided by age (age ≤3 and >3 years, 47.27±7.98 vs. 43.90±8.99 months, χ2=0.002, P=0.963), or by pathology type (epithelial, macrotrabecular and mixed type, 57.01±9.15 vs. 39.63±7.04 vs. 22.00±5.35 months, χ2=4.166, P=0.125), or by vascular involvement (vascular involvement and non-vascular involvement, 33.00±4.82 vs. 51.13±8.23 months, χ2=0.659, P=0.417) (Figure 5A,B,C). In addition, there was no statistical difference in OS between patients with extrahepatic abdominal organs involvement and tumor rupture and patients without extrahepatic abdominal organs involvement and tumor rupture (44.77±6.98 vs. 45.93±9.83 months, χ2=0.002, P=0.966) (Figure 5D).
Discussion
HB is the most common primary hepatic tumor in pediatric population. The incidence of HB has been increasing in the past 30 years, with an annual increase of up to 2.7% (20), which may be related to the improved survival rate in premature and very low birth weight infants (19). Although treatment outcome has improved over the past two decades, the presence of distant metastasis at the time of diagnosis is still the strongest predictor of poor prognosis (15,23). Lung is the most common site of distant metastasis of HB, and there are few reports on the prognosis in HB patients with pulmonary metastasis and extrapulmonary involvement (13). In this study, we aimed to analyze the clinical characteristics and outcome in HB children with pulmonary metastasis and extrapulmonary involvement.
Zhang et al. retrospectively analyzed 102 HB patients from September 2006 to June 2014, and reported that 49 (48.04%) HB patients had distant metastasis (25). Among these 49 patients, 37 (75.51%) had lung metastasis, 10 (20.41%) had vascular metastasis, 17 (34.69%) had intrahepatic metastasis, and 6 (12.24%) had bone metastasis. These results indicated that lung was the most common site of distant metastasis of HB. In addition, Zsiros et al. reported that the patients with vascular involvement and extrahepatic abdominal organs involvement accounting for 39% and 10%, respectively (26). In this study, we retrospectively analyzed 36 HB children with pulmonary metastasis and extrapulmonary involvement, and found that 16 (44.44%) cases had vascular metastasis, 13 (36.11%) cases had brain metastasis, and 10 (27.78%) cases had extrahepatic abdomen involvement. These results indicated that the common sites of HB with extrapulmonary metastasis were blood vessels, brain, and extrahepatic abdominal organs, which consistent with previous reports.
Previous studies had demonstrated that the 5-year survival rate of HB with lung metastasis was about 25–50% (19,20). These results were found to be higher than in our study. In this study, the median survival time of 35 patients who received standardized chemotherapy for more than 6 cycles was 47.16±6.33 months, with a 3-year survival rate of 48.6% and 5-year survival rate of 23.7%. The possible reason was that we included HB patients with pulmonary metastasis and extrapulmonary involvement, which had a relatively poor prognosis.
Previous studies showed that the prognostic factors of HB included distant metastasis, vascular involvement, extrahepatic abdominal organs involvement and tumor rupture, AFP level and PRETEXT stage (15,27). In this study, we found that AFP level, PRETEXT stage and distant metastases had significant impact on survival time, which consistent with previous reports. However, there no statistically significant differences in OS were identified when the study group was divided by vascular involvement or extrahepatic abdominal organs involvement. Considering lung metastasis itself was the main factor affecting prognosis, and the sample size was not large enough, vascular involvement or extrahepatic abdominal organs involvement may have no significant effect on the outcome.
Conclusions
The common sites of extrapulmonary metastasis of HB were blood vessels, brain and extrahepatic abdominal organs. The overall prognosis of HB patients with lung metastasis and extrapulmonary involvement was poor, especially those with PRETEXT stage IV, high AFP level or distant metastases. These results may provide some valuable information for further treatment.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1876
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1876
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1876). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Beijing Tongren Hospital, Capital Medical University (No. 20180212) and informed consent was taken from all participants and their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol 2017;34:192-200. [Crossref] [PubMed]
- Towbin AJ, Braojos Braga FDC, Zhang B, et al. Fractures in children with newly diagnosed hepatoblastoma. Pediatr Radiol 2018;48:581-5. [Crossref] [PubMed]
- McCarville MB, Roebuck DJ. Diagnosis and staging of hepatoblastoma: imaging aspects. Pediatr Blood Cancer 2012;59:793-9. [Crossref] [PubMed]
- Vlajnic T, Brisse HJ, Aerts I, et al. Fine needle aspiration in the diagnosis and classification of hepatoblastoma: Analysis of 21 New Cases. Diagn Cytopathol 2017;45:91-100. [Crossref] [PubMed]
- Fuchs J, Rydzynski J, Von Schweinitz D, et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer 2002;95:172-82. [Crossref] [PubMed]
- Aronson DC, Schnater JM, Staalman CR, et al. Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 2005;23:1245-52. [Crossref] [PubMed]
- Meyers RL, Rowland JR, Krailo M, et al. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 2009;53:1016-22. [Crossref] [PubMed]
- Maibach R, Roebuck D, Brugieres L, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer 2012;48:1543-9. [Crossref] [PubMed]
- Hermann RE, Lonsdale D. Chemotherapy, radiotherapy, and hepatic lobectomy for hepatoblastoma in an infant: report of a survival. Surgery 1970;68:383-8. [PubMed]
- Tiao GM, Bobey N, Allen S, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr 2005;146:204-11. [Crossref] [PubMed]
- Towu E, Kiely E, Pierro A, et al. Outcome and complications after resection of hepatoblastoma. J Pediatr Surg 2004;39:199-202; discussion 199-202. [Crossref] [PubMed]
- Honeyman JN, La Quaglia MP. Malignant liver tumors. Semin Pediatr Surg 2012;21:245-54. [Crossref] [PubMed]
- Hishiki T, Matsunaga T, Sasaki F, et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: report from the JPLT. Pediatr Surg Int 2011;27:1-8. [Crossref] [PubMed]
- Shi Y, Geller JI, Ma IT, et al. Relapsed hepatoblastoma confined to the lung is effectively treated with pulmonary metastasectomy. J Pediatr Surg 2016;51:525-9. [Crossref] [PubMed]
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol 2017;18:122-31. [Crossref] [PubMed]
- Rai P. J HF. Cerebral metastasis of hepatoblastoma: a review. J Pediatr Hematol Oncol 2016;38:279-82. [Crossref] [PubMed]
- Rowland JM. Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol 2002;39:478-83. [Crossref] [PubMed]
- Roebuck DJ, Aronson D, Clapuyt P, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 2007;37:123-32; quiz 249-50. [Crossref] [PubMed]
- Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 2000;36:1418-25. [Crossref] [PubMed]
- Perilongo G, Brown J, Shafford E, et al. Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 2000;89:1845-53. [Crossref] [PubMed]
- Meyers RL, Tiao G, de Ville de Goyet J, et al. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 2014;26:29-36. [Crossref] [PubMed]
- Perilongo G, Maibach R, Shafford E, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 2009;361:1662-70. [Crossref] [PubMed]
- Zsiros J, Maibach R, Shafford E, et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 2010;28:2584-90. [Crossref] [PubMed]
- Committee CA-CAP. Group CMAPO-s. Expert consensus for multidisciplinary Management of Hepatoblastoma (CCCG-HB-2016). Chinese Journal of Pediatric Surgery 2017;38:733-9.
- Zhang Y, Zhang W, Tang S, et al. A single-center retrospective study of pediatric hepatoblastoma. Oncol Lett 2016;12:3919-25. [Crossref] [PubMed]
- Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 2013;14:834-42. [Crossref] [PubMed]
- Hiyama E. Pediatric hepatoblastoma: diagnosis and treatment. Transl Pediatr 2014;3:293-9. [PubMed]


