
A novel guiding tube modified from a Foley catheter for endostapling during robot-assisted pulmonary resection
Introduction
Surgical robot is now increasingly applied to minimally invasive pulmonary resection due to their advantages of high-definition three dimensional vision, tremor filtration and a 7-degree articulation (1-3). Previous studies have indicated sound oncologic results (4) with shorter hospital stays and lower morbidity associated with robotic lobectomy compare to video-assisted thoracoscopic surgery (VATS) and open process (5-7).
Nevertheless, the safety issues relating to minimally invasive thoracic surgery remain a concern (8,9). Significant bleeding caused by vascular injury is dangerous and, in most instances, happened in the early stage of learning curve (10). It is reported that vascular injury result in 29–45% of incidences of conversion to thoracotomy in minimally invasive pulmonary surgery (9-12). Therefore, we proposed a guiding tube modified from a two-way Foley catheter, and described the procedure to use this approach based on our experiences in robot-assisted right upper lobectomy.
Methods
Patients
From July 2018 to June 2019, this guiding method was adopted in a total of 31 patients who underwent RATS (robot-assisted thoracoscopic surgery) lobectomy. Patient demographics and perioperative parameters were collected from the institutional database, and the short-term follow-up were completed by postoperative visits or telephone calls. Descriptive statistics for continuous variables were reported as median (rang) and categoric variables summarized by percentage and frequency. The trial was conducted in accordance with the Declaration of Helsinki. The study was approved by Ethics Committee of West China Hospital of Sichuan University (No. 2020-979) and informed consent was taken from all the patients.
Feature of the guiding tube
As shown in Figure 1, the guiding tube consisted of two parts. A thin head was used to pass through the tunnel and a thick end was used to introduce the anvil jaw of linear stapler. Methods to modify the two-way Foley catheter was shown in Figure 1. We cut off the majority of the thin head of two-way 16Fr Foley catheter at an angle of about 30 to 60 degree and then cut off the side end along with its long axis. With such simple procedure, a guiding tube was created.
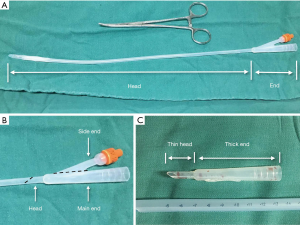
Usage of the guiding tube
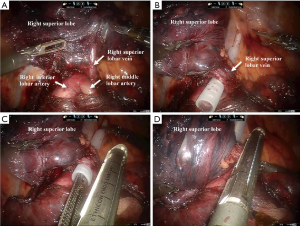
Robotic right upper lobectomy was used an introductory example of how to apply the guiding tube. When the pulmonary tunnel behind the right superior lobar vein was created as illustrated in Figure 2, we intended to divide the right superior lobar vein using a linear stapler (Echelon Flex Powered Stapler 60 mm, Ethicon Endosurgery, USA) through this tunnel. However, this potential tunnel was surrounded by several vessels including pulmonary artery to the right superior lobe, right middle lobe and right inferior lobe, making it difficult and dangerous for the stapler to pass through this tunnel. The guiding tube was applied to guide insertion of a linear stapler. Firstly, the thin head of the guiding tube was inserted in this potential tunnel and then the anvil jaw of the linear stapler was inserted in the thick end of guiding tube. This was then guided by the modified Foley catheter to pass through the tunnel successful and smoothly. Once the linear stapler had successfully passed through the tunnel, the guiding tube was removed from the stapler which was then fired to divided right superior lobar vein.
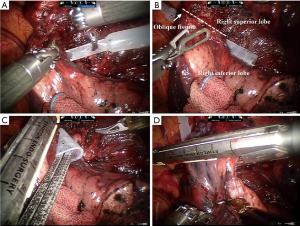
In addition, the guiding tube can also be used to plan a more appropriate direction for stapler to be inserted. As presented in Figure 3, a tunnel under the oblique fissure already created and we intended to divide the oblique fissure with a linear stapler. The stapler was initially orientated in poor direction so that the angle between the stapler and guiding tube was too large to allow the stapler to pass through. The direction of the stapler was adjusted to allow successful insertion of the stapler at a smaller angle.
Results
From July 2018 to June 2019, a total of 31 patients with a median age of 51.5 years old underwent robot-assisted lobectomy in the Department of Thoracic Surgery of West China Hospital, Sichuan University. The demographic characteristics and postoperative short-term outcomes are summarized in Table 1. The median surgical time was 180 min and the median loss of blood was 100 mL. Only one patient was converted to open thoracotomy for silicoanthracotic lymph nodes adhered in hilum of lung. The median duration of chest drainage was 3 days. There was no massive bleeding during and after operation. One patient suffered chylothorax postoperatively and was successfully treated by conservative treatment. The median length of hospital stay was 5 days. There was no mortality in 30 days after hospital discharge.
Table 1
| Characteristics | Patients (n=31) |
|---|---|
| Age (years) | 51.5 [39–74] |
| Sex, n (%) | |
| Male | 10 (32.3) |
| Female | 21 (67.7) |
| BMI (kg/m2) | 23.8 [19.5–30.5] |
| Surgical time (min) | 180 [120–330] |
| Loss of blood (mL) | 100 [20–300] |
| Histological type, n (%) | |
| Squamous carcinoma | 4 (12.9) |
| Adenocarcinoma | 25 (80.6) |
| Inflammatory nodule | 2 (6.5) |
| Duration of chest drainage (day) | 3 [2–25] |
| Complication, n (%) | |
| Chylothorax | 1 (3.2) |
| Pulmonary infection | 1 (3.2) |
| Converting to open thoracotomy | 1 (3.2) |
| Length of hospital stay (day) | 5 [3–45] |
BMI, body mass index.
Discussion
Although minimally invasive surgery has been widely adopted, the safety of minimally invasive pulmonary resection remains a major concern (8,9,13,14). Management of severe intraoperative complication such as vascular injury is generally thought as a weakness of minimally invasive pulmonary resection (4). Once the main vessel is injured during operation, conversion to thoracotomy is an unavoidable consequence in some scenarios (8,10,15,16). Published studies have indicated that adoption of linear stapler might be a potential causes of intraoperative vascular injury on the basis of direct injury and large tension caused by inappropriate direction of passing stapler and surrounding soft tissue that blocked the movement of the stapler (8,17,18).
It seems that robotic lobectomy accompanies less blood loss and lower rate of conversion (5,19). Nevertheless, lack of tactile feedback (7) and unexperienced assistant who stapling vessels are still the potential risk factors for massive bleeding in operation using surgical robot. It has been reported that intraoperative massive bleeding is still the main reason for converting to thoracotomy in robotic pulmonary resection (1,5,7,20).
Though several methods were recommended to treat intraoperative bleeding effectively (9-11), the best way to manage it is to prevent it from happening. Previous study proposed two types of guiding methods for the stapler to prevent major vascular injury during operation (17,18). Lin et al. introduced a Penrose drain tube to guide a stapler in VATS lobectomy (17). It was useful for surgeons to apply such guiding method to pass stapler smoothly. However, the guiding tube modified from Penrose drain tube shared the same diameter, which made it difficult to pass through a narrow tunnel under pulmonary tissue. Yang and colleagues proposed another type of guiding tip modified from a urethral Nélton catheter (18). In this approach, the Nélton tip was placed into the anvil of stapler, and the modified stapler can be self-guided to pass through the tunnel under the pulmonary vessel and soft tissue. This economical tip was effective in reducing the required time for the procedure, however, several deficiencies of this method should be considered. The tip placed in the stapler cannot assist the planning of the direction for the stapler before passing through the tunnel under pulmonary tissue, which may result in high tension between the stapler and pulmonary vessels caused by inappropriate direction of passing stapler. Consequently, massive bleeding would suddenly occur due to the ruptured vessel.
The guiding tube presented in this study consisted of a tiny, flexible head that protects the pulmonary vessels from injury when passing through the tunnel under pulmonary tissue, and a thick end was designed to introduce the anvil jaw of a linear stapler. The conical shape of this guiding tube is novel for guiding stapler to pass through both a wide or narrow tunnel under the vulnerable pulmonary vessel.
In this report, a total of 31 patients underwent RATS lobectomy with this guiding tube in one single medical team of our medical center. There was no conversion to thoracotomy caused by intraoperative vascular injury. However, this report has certain limitations. Only 31 patients were included in this study and so further validation through a larger prospective controlled trial with large sample size is recommended to confirm these findings.
In summary, this modified Foley catheter seems to be a promising guiding method in robotic pulmonary resection.
Acknowledgments
Funding: This study was supported by grants from
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-1492
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/tcr-19-1492). Dr. FW, Dr. HZ, Dr. YZ and Dr. YW report grants from Ministry of Science and Technology of the People’s Republic of China and from Chengdu Municipal Bureau of Science and Technology during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of West China Hospital of Sichuan University (No. 2020-979) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veronesi G, Park B, Cerfolio R, et al. Robotic resection of Stage III lung cancer: an international retrospective study. Eur J Cardiothorac Surg 2018;54:912-9. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin 2014;24:157-62. vi. [Crossref] [PubMed]
- Mahieu J, Rinieri P, Bubenheim M, et al. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac Cardiovasc Surg 2016;64:354-62. [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Augustin F, Maier HT, Weissenbacher A, et al. Causes, predictors and consequences of conversion from VATS to open lung lobectomy. Surg Endosc 2016;30:2415-21. [Crossref] [PubMed]
- Byun CS, Lee S, Kim DJ, et al. Analysis of Unexpected Conversion to Thoracotomy During Thoracoscopic Lobectomy in Lung Cancer. Ann Thorac Surg 2015;100:968-73. [Crossref] [PubMed]
- Vallance A, Tcherveniakov P, Bogdan C, et al. The evolution of intraoperative conversion in video assisted thoracoscopic lobectomy. Ann R Coll Surg Engl 2017;99:129-33. [Crossref] [PubMed]
- Gonzalez-Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgerydagger. Eur J Cardiothorac Surg 2016;49:i17-24. [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Aragon J, Perez Mendez I. From open surgery to uniportal VATS: asturias experience. J Thorac Dis 2014;6:S644-9. [PubMed]
- Bertolaccini L, Davoli F, Pardolesi A, et al. Conversion due to vascular injury during video-assisted thoracic surgery lobectomy: A multicentre retrospective analysis from the Italian video-assisted thoracic surgery group registry. Eur J Surg Oncol 2019;45:857-62. [Crossref] [PubMed]
- Lin MW, Lee JM, Lee YC. Penrose drain tube as a guide for endostaplers during lobectomy via video-assisted thoracoscopic surgery. Thorac Cardiovasc Surg 2010;58:184-5. [Crossref] [PubMed]
- Yang SM, Chen JS, Lee JM. Handmade guiding tip using a Nelaton tube for endostapler application to pulmonary vessels. Thorac Cardiovasc Surg 2014;62:725-7. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]


