
HPV16 E6 regulates the proliferation, invasion, and apoptosis of cervical cancer cells by downregulating miR-504
Introduction
Cervical cancer is one of the most common malignant tumors in women, with more than 500,000 confirmed cases every year (1). More than 85% of cases occur in economically underdeveloped areas because of the lack of effective screening and treatment strategies (2). However, the essential mechanism underlying the development of cervical cancer remains elusive. Studies have shown that human papillomavirus (HPV) 16 infection is a necessary condition for the pathogenesis and development of cervical cancer. The E6 protein is expressed by the HPV16 E6 gene and promotes malignant phenotype transformation, which is an important mechanism for the occurrence and development of cervical cancer (3,4).
MicroRNAs (miRNAs) are considered important tumor regulators and have been reported as oncogenes or tumor suppressor genes in the occurrence and development of cervical cancer by influencing cell biologic function (5,6).
Cumulative studies have shown that the abnormal expression of miRNAs in cervical cancer is closely related to HPV16 infection, and the targeted interference of HPV16 E6 expression can affect miRNA expression (7). MicroRNA-504 (miR-504) is a miRNA that is abnormally expressed in osteosarcoma, glioblastoma, and nasopharyngeal carcinoma; it has been reported as an oncogene or tumor suppressor gene, as it regulates the biologic behavior of tumor cells (8-10). The expression of miR-504 in cervical cancer has been found to be negatively correlated with HPV infection (11), while the role and underlying mechanism remain elusive. In the present study, we hypothesize that HPV16 E6 may promote the development of cervical cancer by regulating the expression of miR-504.We observed the effect of HPV16 E6 on the expression of miR-504 in cervical cancer cells, and analyzed whether HPV16 E6 affect proliferation, invasion, and apoptosis in cervical cancer cells by regulating the expression of miR-504.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2913).
Methods
Materials
Human cervical cancer cells (SiHa) were preserved at our laboratory. HyClone penicillin-streptomycin 100× solution (Cytiva), Dulbecco’s modified Eagle’s medium (DMEM; Gibco), fetal bovine serum (FBS; Hangzhou Sijiqing Biological), TRIzol (Thermo Fisher Scientific), Lipofectamine 2000 (Invitrogen), HPV16 E6 antibody (Santa Cruz), GAPDH antibody (Santa Cruz), matrigel matrix (BD Biosciences), -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit, (Sigma-Aldrich), annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Beijing Solebaon Technologies), sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE; Beijing Solebaon Technologies) preparation kit, bicinchoninic acid (BCA) protein concentration determination kit (Beijing Solebao Technologies), pcDNA3.1-HPV16 E6 overexpression vector (Shanghai Jima Biological), and miR-504 mimic and its negative control (Guangzhou RuiboBiological) were used in the present study.
Cell culture and transfection
SiHa cells were cultured with DMEM containing penicillin-streptomycin and 10% FBS in a 5% CO2 incubator at 37 °C. The cells were divided into four groups: (I) empty vector group (transfected with pcDNA3.1 empty vector); (II) E6 overexpression group (transfected with pcDNA3.1–HPV16 E6 overexpression vector); (III) E6 overexpression + miR-NC group (co-transfection with pcDNA3.1-HPV16 E6 overexpression vector and miR-NC; and (IV) E6 overexpression + miR-504 group (co-transfection with pcDNA3.1-HPV16 E6 overexpression vector and miR-504 mimic). SiHa cells were seeded into six-well cell culture plates with 1×106 cells/well, and were cultured in a 5% CO2 incubator at 37 °C. The cells were transfected with pcDNA3.1-HPV16 E6 overexpression vector, pcDNA3.1 empty vector, miR-504 mimic and miR-NC by using Lipofectamine 2000 according to the manufacturer’s instructions. Cells were changed with fresh medium 5 hours after transfection, and the cell lysates in each group were collected after 48hours of transfection.
Real-timepolymerase chain reaction (PCR)
Total RNA was extracted with TRIzol reagent; cDNA was synthesized using a reverse transcription kit according to the manufacturer’s instruction. The Applied Biosystems 7500 Real-Time PCR System was used for the real-time PCR. The reaction conditions were as follows: pre-denaturation at 95 °C for 3 minutes, denaturation at 95 °C for 40 seconds, annealing at 56 °C for 30 seconds, and extending for 30 seconds at 72 °C for 40 cycles. Using GAPDH or U6 as the internal reference, HPV16 E6 mRNA and miR-504 relative expression levels in SiHa cells were calculated by 2–△Ct method. The experiment was repeated three times. The primer sequences are shown in Table 1.
Table 1
| Gene | Upstream primer | Downstream primer |
|---|---|---|
| HPV16 E6 | 5'-gagcgaccagaagttaccca-3' | 5'-aaatcccgaagcaagcaaaagtca-3' |
| GAPDH | 5'-tgggtgtgaaccaccgagaa-3' | 5'-ggcatggactgtggtcatgga-3' |
| miR-504 | 5'-gcgggcgagagacctgtctgcac-3' | 5'-atccaggtgcag-ccgag-3' |
| U6 | 5'-gccttcggcagcacatactat aaaaat-3' | 5'-cgcttcacgagaatttgcgtgtca-3' |
HPV, human papillomavirus; miR-504, microRNA-504.
Western blotting
Cell lysates were collected 48 hours after transfection, and the protein concentration was determined by BCA method. The protein sample was boiled for 5 minutes in a water bath. The denatured protein was separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. After blocking with 5% skimmed milk powder for 2 hours at room temperature, the membrane was incubated with the primary antibody (1:500) overnight at 4 °C. After washing with phosphate-buffered saline containing 0.05% Tween-20 (PBST) three times, the membrane was incubated with t horseradish peroxidase-labeled secondary antibody (1:2,000) at 37 °C for 1 hour. After washing with PBST three times, the membranes were developed with the chemiluminescent agent. The expression level of the protein was detected using a gel imaging analysis system (Gel Doc XR+; Bio-Rad). The experiment was repeated three times.
MTT assay
SiHa cells were seeded into a 96-well cell culture plate with 5×103 cells/well, and incubated in a 5% CO2 incubator at 37°C.The cells were divided into four: (I) empty vector group; (II) E6 overexpression group; (III) E6 overexpression + miR-NC group; and (IV) E6 overexpression+miR-504 group. The cells were incubated with MTT assay (10 µL/well) for 4 hours at 48, 72, and 96 hours, respectively. The cells were then added to dimethyl sulfoxide (DMSO) (150 µL/well) in a shaker for 15 minutes. The optical density value at 490 nm was detected by using an enzyme-labeled instrument (iMark; Bio-Rad). The experiment was repeated three times.
Transwell assay
Matrigel matrix gel (50 mg/L), diluted at a ratio of 1:8, was spread onto the upper surface of the membrane and dried at 4 °C. SiHa cell suspensions (1×105 cells/mL) were added into the upper chamber, with 100 µL/well and 600 µL medium containing 10% FBS added into the lower chamber. After 24 hours of culture, the cells were removed and fixed with4% paraformaldehyde. The cells were dyed with 0.5% crystal violet. After washing, the number of cells penetrating the membrane was observed and counted under a light microscope. The results were expressed by the mean number of cells randomly selected in five visual fields. The experiment was repeated three times.
Cell apoptosis
SiHa cells in each group were collected into a centrifuge tube; each sample contained 1×106 cells. The medium was discarded after centrifugation at 800 ×g/minute for 5 minutes. The cells were then washed twice with phosphate-buffered saline (PBS); 200 µL PBS was added, and the cells were resuspended in the mixture. A total of5 µL annexin V-FITC and 5 µL PI were added to the cells and incubated at room temperature for 15 minutes under dark conditions. The apoptotic rate of SiHa cells was detected by using a flow cytometer (FACSC alibur; BD Biosciences). The experiment was repeated three times.
Statistical analysis
SPSS version 22.0 was used for the statistical analysis. Each assay was conducted triplicately. The measurement data was expressed using mean ± standard deviation. Single-factor analysis of variance was used to analyze the differences among groups, and Q-test was used to analyze the differences between two groups. P<0.05 indicated a statistically significant difference.
Results
Effect of HPV16E6 on the expression of miR-504 in SiHa cells
As shown in Figure 1, the expression levels of the HPV16 E6 mRNA (Figure 1A) and protein (Figure 1B) in E6 overexpression cells were significantly increased compared to the control cells (empty vector group). In addition, the expression level of miR-504 was significantly decreased in E6 overexpression cells compared to the control cells (P<0.05) (Figure 1C).
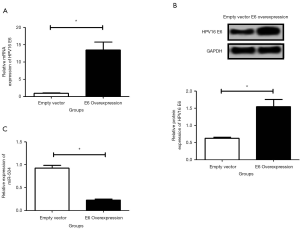
Expression of miR-504 in SiHa cells of each group
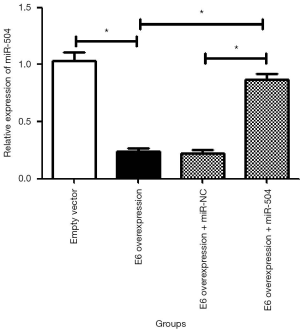
As shown in Figure 2, the overexpression of E6 reduced the expression level of miR-504 in SiHa cells; the difference was statistically significant (P<0.05). The overexpression of miR-504 with the miR-504 mimic significantly reversed the downregulation of miR-504 in E6 overexpression SiHa cells (P<0.05).
As shown in Table 2, MTT assay showed that the overexpression of E6 significantly increased the proliferation of SiHa cells (P<0.05). The overexpression of miR-504 reversed the role of HPV16 E6 on proliferation in E6 overexpression SiHa cells; the difference was statistically significant (P<0.05).
Table 2
| Groups | 48 hours | 72 hours | 96 hours |
|---|---|---|---|
| Empty vector | 0.28±0.02 | 0.50±0.03 | 0.82±0.06 |
| E6 overexpression | 0.40±0.03* | 0.83±0.05* | 1.35±0.11* |
| E6 overexpression + miR-NC | 0.41±0.03 | 0.85±0.05 | 1.29±0.08 |
| E6 overexpression + miR-504 | 0.33±0.02**,*** | 0.68±0.04**,*** | 0.97±0.06**,*** |
| F | 52.154 | 126.240 | 90.432 |
| P value | <0.000 | <0.000 | <0.000 |
*, P<0.05 compared with empty vector group; **, P<0.05 compared with E6 overexpression group; ***, P<0.05 compared with E6 overexpression + microRNA (miR)-NC group.
HPV16 E6 regulates invasion of SiHa cells via miR-504
As shown in Figure 3, the overexpression of E6 significantly increased the invasion of SiHa cells (P<0.05), as shown in the Transwell assay. The overexpression of miR-504 reversed the role of HPV16 E6 on invasion in E6 overexpression SiHa cells; the difference was statistically significant (P<0.05).
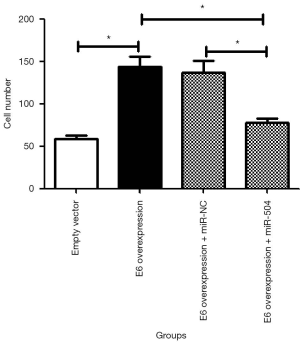
HPV16 E6 regulates apoptosis of SiHa cells via miR-504
As shown in Figure 4, the overexpression of E6 significantly reduced apoptosis of SiHa cells (P<0.05). The overexpression of miR-504 reversed the role of HPV16 E6 on apoptosis in E6 overexpression SiHa cells; the difference was statistically significant (P<0.05).
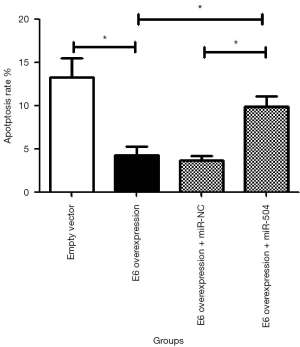
Discussion
The HPV16 E6 gene is located at position 83-559 of the HPV16 genome and contains 477 nucleotides. The E6 protein is encoded by the E region gene and consists of 151 amino acids and two zinc finger structures. It can degrade p53 by binding to E6-related proteins, and plays an important role in cell cycle, apoptosis, and malignant phenotype transformation (12). HPV16 E6 can promote the occurrence and development of cervical cancer by regulating the function of tumor cells. Previously published studies have found that HPV16 E6 is highly expressed in cervical cancer, and its expression level is closely related to the grade of cervical lesions (13). HPV16 E6 can induce cancer cell proliferation and inhibit cell apoptosis by upregulating MIF expression (14). HPV16 E6 promotes the migration and invasion of cervical cancer cells by downregulating the expression of NHERF1 (15). In the present study, we found that the overexpression of HPV16 E6 significantly enhanced the proliferation and invasion of SiHa cells, whereas apoptosis was significantly reduced. The results are consistent with those of previous studies (14-16), and further verify that HPV16 E6 can promote proliferation and invasion, and inhibit apoptosis, in cervical cancer cells.
miRNAs are a class of endogenous non-coding RNAs that can regulate gene expression by inhibiting target gene transcription or protein translation; they affect cell proliferation, invasion, and apoptosis, as well as other biologic processes. miRNAs are closely related to the occurrence and development of many tumors, including HPV-related tumors. HPV infection is an important carcinogenic mechanism by regulating the expression of various miRNAs. Zang et al. reported that miR-125b has a low expression in HPV16 E6(+) esophageal cancer tissues, and is negatively correlated with HPV16 E6 expression (16). HPV16 E6 overexpression could activate the Wnt/β-catenin signaling pathway by reducing the expression of miR-125b in esophageal cancer cells, and promote the occurrence of esophageal cancer. Hufbauer et al. found that miR-3194-5p and miR-1281 were upregulated in HPV16 E6(+) oropharyngeal squamous cell carcinoma (17). HPV16 E6 could increase the number of transitional cancer stem cells by regulating the expression of miR-3194-5p and miR-1281. There are also miRNAs closely related to HPV16 infection in cervical cancer. The expression level of miR-34a in cervical cancer was found to be related to HPV16 infection; miR-34a could be used to evaluate and predict the prognosis of HPV infection (18). Peta et al. noted that miR-146a-5p was downregulated in HPV16 E6(+) cervical cancer, whereas HPV16 E6 could upregulate KMD2b expression by regulating the c-myc/miR-146a-5p axis and promote the proliferation and migration of cervical cancer cells (19).
miR-504 is closely related to tumorigenesis and development. It has been reported that miR-504 is abnormally expressed in non-small cell lung cancer, glioma, and breast cancer, and acts as an oncogene or tumor suppressor gene by regulating cell biologic function (20-22). In a previously published study, the expression of miR-504 was found to be downregulated in cervical cancer and related to HPV infection; however, the relationship between HPV16 E6 and miR-504 and the role of miR-504 in cervical cancer were not clear (11). In the present study, we found that the overexpression of HPV16 can downregulate the expression of miR-504 in SiHa cells, indicating that miR-504 may play an important role in the development of cervical cancer induced by HPV16 E6. In addition, transfection with the miR-504 mimic can reverse the role of HPV16 E6 on the proliferation, invasion, and apoptosis of SiHa cells. The results indicate that miR-504 could inhibit proliferation and invasion, and induce apoptosis, of SiHa cells. HPV16 E6 could promote the malignant development of SiHa cells by downregulating the expression of miR-504.
However, there are some limitations in the current study. Firstly, the in vivo experiments are lacking, which will be conducted in the next research work. Secondly, the in-depth mechanism has not been elusive, which is more meaningful.
In conclusion, HPV16 E6 can promote the proliferation and invasion, and inhibit the apoptosis, of SiHa cells by downregulating the expression of miR-504. The role of HPV16 E6 on the development of cervical cancer in vitro was discussed; however, further research is required to further verify this finding in animal experiments, which will help elucidate the carcinogenic mechanism of HPV16 E6 in cervical cancer.
Acknowledgments
Funding: The present study was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2913
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2913
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2913). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burk RD, Harari A, Chen Z. Human papillomavirus genome variants. Virology 2013;445:232-43. [Crossref] [PubMed]
- Li H, Yang Y, Zhou H, et al. Clinicopathologic characteristics and survival analysis in stage IVB cervical cancer with hematogenous metastasis. Transl Cancer Res 2019;8:1217-23. [Crossref]
- Han L, Maimaitiming T, Husaiyin S, et al. Comparative study of HPV16 integration in cervical lesions between ethnicities with high and low rates of infection with high-risk HPV and the correlation between integration rate and cervical neoplasia. Exp Ther Med 2015;10:2169-74. [Crossref] [PubMed]
- Marongiu L, Godi A, Parry JV, et al. Human papillomavirus type 16 long control region and E6 variants stratified by cervical disease stage. Infect Genet Evol 2014;26:8-13. [Crossref] [PubMed]
- Cao Q, Wang N, Ren L, et al. miR-125a-5p post-transcriptionally suppresses GALNT7 to inhibit proliferation and invasion in cervical cancer cells via the EGFR/PI3K/AKT pathway. Cancer Cell Int 2020;20:117. [Crossref] [PubMed]
- Zhu J, Han S. miR-150-5p promotes the proliferation and epithelial-mesenchymal transition of cervical carcinoma cells via targeting SRCIN1. Pathol Res Pract 2019;215:738-47. [Crossref] [PubMed]
- Lin M, Xue X, Liang S, et al. MiR-187 overexpression inhibits cervical cancer progression by targeting HPV16 E6. Oncotarget 2017;8:62914-26. [Crossref] [PubMed]
- Cai Q, Zeng S, Dai X, et al. miR-504 promotes tumour growth and metastasis in human osteosarcoma by targeting TP53INP1. Oncol Rep 2017;38:2993-3000. [Crossref] [PubMed]
- Liu Q, Guan Y, Li Z, et al. miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt/β-catenin pathway. J Exp Clin Cancer Res 2019;38:358. [Crossref] [PubMed]
- Zhao L, Tang M, Hu Z, et al. miR-504 mediated down-regulation of nuclear respiratory factor 1 leads to radio-resistance in nasopharyngeal carcinoma. Oncotarget 2015;6:15995-6018. [Crossref] [PubMed]
- Guo Y, Tao M, Jiang M. MicroRNA-454-3p inhibits cervical cancer cell invasion and migration by targeting c-Met. Exp Ther Med 2018;15:2301-6. [Crossref] [PubMed]
- Zhou M, Chen X, Wu J, et al. MicroRNA-143 regulates cell migration and invasion by targeting GOLM1 in cervical cancer. Oncol Lett 2018;16:6393-400. [Crossref] [PubMed]
- Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human Papillomavirus 16 E6 Antibodies in Individuals without Diagnosed Cancer: A Pooled Analysis. Cancer Epidemiol Biomarkers Prev 2015;24:683-9. [Crossref] [PubMed]
- Andersson S, Alemi M, Rylander E, et al. Uneven distribution of HPV 16 E6 prototype and variant (L83V) oncoprotein in cervical neoplastic lesions. Br J Cancer 2000;83:307-10. [Crossref] [PubMed]
- Wang Q, Ran S, Zhao C, et al. HPV16 E6 promotes cervical cancer cell migration and invasion by downregulation of NHERF1. Int J Cancer 2019;144:1619-32. [Crossref] [PubMed]
- Zang B, Huang G, Wang X, et al. HPV-16 E6 promotes cell growth of esophageal cancer via downregulation of miR-125b and activation of Wnt/β-catenin signaling pathway. Int J Clin Exp Pathol 2015;8:13687-94. [PubMed]
- Hufbauer M, Maltseva M, Meinrath J, et al. HPV16 increases the number of migratory cancer stem cells and modulates their miRNA expression profile in oropharyngeal cancer. Int J Cancer 2018;143:1426-39. [Crossref] [PubMed]
- Yu Y, Zhang Y, Zhang S. MicroRNA-92 regulates cervical tumorigenesis and its expression is upregulated by human papillomavirus-16 E6 in cervical cancer cells. Oncol Lett 2013;6:468-74. [Crossref] [PubMed]
- Peta E, Sinigaglia A, Masi G, et al. HPV16 E6 and E7 upregulate the histone lysine demethylase KDM2B through the c-MYC/miR-146a-5p axys. Oncogene 2018;37:1654-68. [Crossref] [PubMed]
- Ye MF, Zhang JG, Guo TX, et al. MiR-504 inhibits cell proliferation and invasion by targeting LOXL2 in non small cell lung cancer. Biomed Pharmacother 2018;97:1289-95. [Crossref] [PubMed]
- Cui R, Guan Y, Sun C, et al. A tumor-suppressive microRNA, miR-504, inhibits cell proliferation and promotes apoptosis by targeting FOXP1 in human glioma. Cancer Letters 2016;374:1-11. [Crossref] [PubMed]
- Jin Z, Jin RH, Ma C, et al. expression level of miR-504 can differentiate between glioblastoma multiforme and solitary brain metastasis of non-small cell lung carcinoma. J BUON 2017;22:474-80. [PubMed]
(English Language Editor: R. Scott)


