
Emerging chemotherapy agents in lung cancer: nanoparticle therapeutics for non-small cell lung cancer
Introduction
Since the late 1990s, platinum-based doublet chemotherapy has been the standard of care for treating non-small cell lung cancer (NSCLC) patients without actionable mutations. ECOG 15-94 compared four regimens (cisplatin/gemcitabine, cisplatin/docetaxel, cisplatin/paclitaxel and carboplatin/paclitaxel) among more than 1,200 patients and found equal efficacy, with all four regimens leading to survival rates of 33% at 1 year and 11% at 2 years in advanced NSCLC (stage IIIB-IV) (1). However, these regimens have significant toxicity, and their benefit is limited to patients with good performance status (ECOG 0-1). Patients who are elderly or unfit (ECOG ≥2) have shorter survival and more chemotherapy-related toxicity (2). Often times, these patients are not offered 1st line systemic chemotherapy due to the unfavorable risk-benefit ratio. Another limitation to platinum doublet chemotherapy is its limited efficacy in refractory disease. While targeted therapy [EGFR (3), ALK (4) inhibitors], and immunotherapy [anti-CTLA-4 (5), anti-PD-1 (6,7)], have led to improved survival and response rates in NSCLC, there remain a significant proportion of patients who do not benefit from these strategies, or whom cannot tolerate them.
To meet these needs, nanoparticle delivery systems present a novel approach for delivering cytotoxic drugs in the treatment of NSCLC, both with higher efficacy and lower toxicity. Abraxane, or albumin-bound nanoparticle paclitaxel, is the first nanoparticle therapy to be FDA-approved for use in NSCLC, based on its improved outcomes and decreased adverse events (8,9). This drug is likely just the first of many, as nanotechnology drug delivery continues to be refined and further utilized. In this review, we discuss emerging nanoparticle therapies for NSCLC. We will first provide an overview of nanoparticle therapies, then provide specific examples of novel agents which have produced promising data (Table 1).
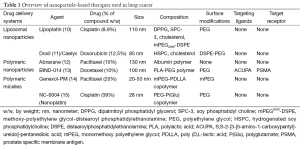
Full table
Nanoparticle therapies
The advent of nanotechnology has brought with it the potential for nanoparticle therapeutics in cancer therapy. Nanoparticles, or nanomaterials, by definition measure 1-100 nm in at least one dimension (16,17). It should be noted that nanoparticle systems may be used for various medical purposes [e.g., imaging (18), immune adjuvants (19)] in addition to therapeutics, and while these agents may be larger, up to 200-500 nm in size, this does not necessarily impact their functionality or detract from their size-dependent properties. Nanoparticles may be engineered as the drug delivery carrier, or the drug itself can be engineered at a nanoscale, in which case the drug serves as its own “carrier” (20-22). Nanomaterials used in cancer nanotherapeutics include lipids, polymers, dendrimers, organometallic and carbon based materials (23).
Nanotechnology drug delivery systems offer several advantages compared to standard chemotherapy. Due to their size dependent properties and additional modifications, nanoparticle drugs can be designed to achieve prolonged circulation times, greater stability, improved intratumoral accumulation and concentration, and decreased toxicity to normal tissues. As such these nanoparticle therapeutics represent more effective therapies with diminished side effects.
Nanoparticles have four particularly unique characteristics (Table 2), thanks to their size and relatively large surface area (up to 1,000 m2/g): (I) large “payload”, or the ability to carry large amounts of drug; (II) multivalent ligand binding, as higher density of targeting moieties leads to higher affinity binding; (III) combination therapeutics, as their large surface to mass ratio can accommodate multiple drugs simultaneously; and (IV) bypass of multidrug resistance mechanisms involving cell surface protein pumps, e.g., P-glycoprotein (24).

Full table
Physical characteristics of nanoparticles
The ideal nanoparticle drug delivery systems range in size from 10-100 nm (Figure 1). Nanoparticles larger than 10 nm and greater than 50 kDa will avoid leakage into capillaries and removal by single-pass renal clearance (25,26). Nanoparticles smaller than 100 nm will escape capture by macrophages in the reticuloendothelial system (RES) (27), where sinusoids in the spleen and the fenestra of Kupffer cells in the liver range from 150-200 nm (28). Furthermore, tumor blood vessels possess wide gap junctions measuring 100-600 nm in size, so nanoparticles smaller than 100 nm will optimally penetrate and accumulate into tumor tissues (29-31).
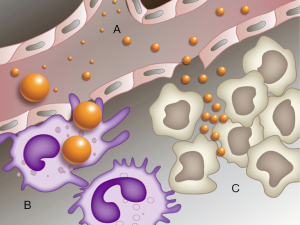
Nanoparticle surfaces can also be modified to optimize their efficacy and targeting. Nanoparticles with hydrophobic surfaces are prone to macrophage capture by the RES (32,33). Thus, modifications to create more hydrophilic surfaces will help evade removal by the RES. Polyethylene glycol (PEG) is the most widely used modification to render nanoparticles more hydrophilic (32). PEGylation of nanoparticle surfaces prevents opsonization, blocking RES capture, and leading to improved circulation time and tumor site accumulation. Alternatively, nanoparticles can also be formed using block copolymers with both hydrophilic and hydrophobic domains (34). Neutral or negatively charged nanoparticle surfaces are also preferred, because positively charged nanoparticles are more readily taken up by the RES (35).
The shape and flexibility of nanoparticles can also be adjusted to improve circulating time. Spherical shaped nanoparticles are more likely to maintain streamlined movement through a vessel (36,37). Soft, flexible nanoparticles are less susceptible to macrophage capture (38,39).
Passive targeting (EPR)
Passive targeting is a size dependent process that capitalizes both on the size and surface characteristics of nanoparticles, as well as the unique features of tumor vessel pathophysiology. Nanoparticles sized 10-100 nm and with the appropriate surface characteristics will evade capture by the RES. As a result, they are retained in the circulation for longer periods and have a higher likelihood of reaching tumor sites. Tumor tissues, meanwhile, are characterized by rapid cell proliferation, active neovascularization and an imbalance of proangiogenic growth factors. Vascular endothelial growth factor (VEGF), bradykinin (40), prostaglandins (40), nitric oxide (40), peroxynitrite (ONOO−), and matrix metalloproteinases (41), all increase the vascular permeability of tumors (42). The end result is an irregular, highly distorted vasculature with wide, leaky gap junctions that are prone to extravasation by nanoparticles smaller than 100 nm in size and with a molecular weight above 50 kDa (43). Because these tumor tissues have poor lymphatic drainage, nanoparticles will be retained in these intratumoral sites for prolonged periods (29,44). This combined effect, whereby nanoparticles exploit the leaky vasculature and impaired lymphatic drainage of tumors, is known as the enhanced permeability and retention effect (EPR) (29). The EPR effect produces higher intratumoral drug concentrations and simultaneously decreased systemic exposure to normal organs; this is a unique advantage of nanoparticle delivery systems and critical to their success in cancer therapy.
Another strategy to improve passive targeting of nanoparticles is via pH-sensitive release (45,46), whereby the cytotoxic drug payload is only delivered upon reaching specific conditions found in the tumor microenvironment. For example, pH-sensitive liposomes are designed to remain stable at physiologic pH, but will degrade and release their active agent at a lower pH. Because tumor cells utilize glycolysis for additional energy production (47), they have an acidic environment, and pH-sensitive liposomes will only activate at this specific location (46).
Active targeting
While passive targeting localizes nanoparticle drugs to intratumoral sites via the EPR effect, it does not ensure intracellular uptake. Active targeting is a strategy to further improve nanoparticle targeting within the cancer cells themselves. Nanoparticles are modified with specific, surface-bound ligands which allow the drug to home with high affinity to corresponding receptors expressed on the cancer cell. Ligand binding to the cancer cell surface receptor triggers receptor-mediated endocytosis, which internalizes the drug deep into the cell. This is an important strategy, because the ability to bypass membrane efflux pumps may allow nanoparticles to overcome drug resistance.
Various moieties, such as peptides, aptamers, proteins, and antibodies, can be added to the surface of nanoparticles as ligands to facilitate conjugation with cancer cell receptors. Examples of cancer cell receptors that have been studied as targets for nanoparticles in lung cancer include the folate receptor (48), EGFR (49,50), transferrin receptor (51), epithelial cell adhesion molecules (EpCAM) (52), interleukin-4 receptor (53), fibroblast activation protein alpha (FAPα) (54), and prostate specific membrane antigen (PSMA) (13).
Preclinical studies suggest that active targeting improves antitumor efficacy as a result of enhanced cellular internalization of cytotoxic agents, rather than increased tumor accumulation (55). This strategy allows not only the further specific targeting of nanoparticle therapeutics, but also decreased toxicity to non-tumor tissue sites. With improved tumor targeting, there will be less drug leakage, less localization of drug at normal tissues, and less degradation or clearance of drug. At the clinical level, this should result in less toxicity for the patient, and allow for higher therapeutic doses to be tolerated.
Overcoming drug resistance
Multidrug resistance in cancer cells is commonly due to over expression of broad spectrum drug efflux pumps in the cellular membrane which non-specifically transport drugs out of the cell, and thereby lower intracellular drug concentrations (56). This leads to tumor cells with resistance to multiple drugs via the same mechanism.
The most prominent family of drug efflux pumps is the ATP-binding cassette (ABC) transporter family of efflux pumps. Among these, the first described drug efflux pump was P-glycoprotein (Pgp) (57,58), the end product of the ABCB1 (or MDR1) gene (59). Pgp is a transporter of cationic lipophilic substances, and may confer resistance to multiple classes of anticancer drugs, including anthracyclines (60), vinca alkaloids (61,62), camptothecins (63), taxanes (62), and epothilones (64). Studies in both small cell and NSCLC have shown increased MDR1 expression in up to 15-50% of tumor samples (65,66).
The multidrug-resistance associated protein (MRP1, or ABCC1) gene codes for a similar ABC transporter that is also associated with multi-drug resistance (67). MRP1 is a transporter of organic ions associated with resistance to anthracyclines (67,68), antimetabolites (69), vinca alkaloids (67,70), topoisomerase inhibitors (71), and alkylating agents (72). MRP1 is ubiquitously expressed in normal lung tissues; in NSCLC, MRP1 expression was found in 100% of samples, with 30% of samples showing increased MRP1 expression (65,73).
Unlike low molecular weight drugs less than 1 nm in diameter, nanoparticles are 1-2 magnitude larger, and thus more likely to undergo intercellular uptake via endocytosis. These drugs are taken up via endo-lysosomal trafficking, into lysosomes, and are taken deep into cells, beyond the reach of drug efflux pumps. In doing so, these nanomedicines may potentially overcome multidrug resistance due to membrane pumps in cancer cells (74). The combined use of multiple drugs within a single nanoparticle may also provide another means for overcoming multidrug resistance going forward (75,76). Combination nanoparticle therapy can act on multiple mechanisms leading to synergism and lesser toxicity through decreased doses compared to single drug regimens (76).
Nanoparticle drug delivery systems
Various nanomaterials have been utilized in cancer therapeutics, including: lipids, polymers, viruses, carbon nanotubes and organometallic compounds. For the purposes of this review, we will only describe liposomes, polymeric nanoparticles and polymeric micelle based delivery systems. Other nanomaterial delivery systems are described elsewhere and beyond the scope of this review.
Liposomes
Lipid-based drug delivery systems, also known as liposomes, are closed spherical structures where amphiphilic phospholipids and cholesterol form one or more lipid bilayers surrounding an aqueous core (77). This liposomal structure allows storage of hydrophilic drugs within the aqueous core, or for hydrophobic drugs to be associated within the lipid bilayers. By improving the solubility of cytotoxic agents, liposomes extend drug circulation times and increase the chance of intratumoral uptake. Cytotoxic agents designed with liposomal formulations include anthracyclines (78-80), platinums (81), camptothecins (82), vinca alkaloids (83), and antimetabolites (84-86).
Doxil
StealthTM PEGylated liposomal doxorubicin, also known as Doxil (US), or Caelyx (EU), was among the first approved nanoparticle chemotherapies, with FDA indications for ovarian cancer, AIDS-related Kaposi sarcoma and multiple myeloma. Doxil has been shown to have significantly less cardiotoxicity compared to standard doxorubicin (87-89). A phase I study demonstrated Doxil’s activity as a single agent in platinum pretreated advanced or metastatic NSCLC (78); among 17 patients, there was 1 PR (5.8%) and 5 SD (29.4%), with median TTP 9.5 weeks and median survival 18.6 weeks. Because of its relatively low toxicity, Doxil 20 mg/m2 IV was combined in a triplet regimen with docetaxel 50 mg/m2 IV and gemcitabine 1,000 mg/m2 IV on a 2 week cycle, for 18 patients with treatment-naïve, advanced NSCLC (17 stage IV, 1 stage III); this triplet regimen resulted in 1 CR (5%) and 6 CR/PR (33%) (90). Patients received supportive growth factors and amifostine to help reduce toxicity; there were no cases of grade III-IV toxicity.
Doxil has been studied as a radiosensitizing agent for definitive therapy of NSCLC. In a phase I dose escalation study among 15 NSCLC patients, an MTD of Doxil 25 mg/m2 every 2 weeks for three cycles concurrently with conventional fractionated radiotherapy resulted in a CR rate of 21% (79). In a subsequent Phase I/II dose escalation study among 15 patients with stage IIIB NSCLC, the combination of Doxil 25 mg/m2 IV every 2 weeks with docetaxel 30 mg/m2 IV weekly during conventional fractionated radiotherapy (total 64 Gy) achieved 6 CR (40%) and 13 CR + PR (87%) (91). Supportive amifostine was administered to all patients; toxicity included grade I neutropenia in five patients (20%), grade II anemia in three patients (12%), and grade III esophagitis in nine patients (36%). The combination of Doxil with cisplatin concurrently with conventional radiation was studied in a phase I of 18 patients (9 squamous cell lung cancer, 9 squamous cell head and neck). Among all patients, there were 6 CR (33%) and 10 PR (55%) (92). A fourth study combined Doxil 20 mg/m2 every 2 weeks for three cycles with vinorelbine during hypofractionated accelerated radiotherapy (15 fractions of 3.5 Gy over 4 consecutive weeks, with 1 week split after the 10th fraction, for a total of 65.6 Gy). All patients received supportive growth factors and amifostine. Among 14 patients, there were 9 PR (64.2%), 3 with minimal response (21.4%), and 2 SD (14.3%) (93).
Doxil has been assessed as salvage therapy for small cell lung cancer. In a Phase II study of Doxil 50 mg/m2 IV every 4 weeks among 14 patients with recurrent small cell lung cancer, there were 3 SD (21.4%), but no PR/CR (94). Doxil 30 mg/m2 was subsequently combined with vincristine 1.2 mg/m2 and cyclophosphamide 750 mg/m2, every 3 weeks among 30 patients with recurrent SCLC, producing 3 PR (10%) and 15 SD (50%) (95). A Phase II study treated 26 patients with relapsed SCLC using Doxil 15 mg/m2 and irinotecan 125 mg/m2 every 2 weeks, resulting in 4 PR (12.9%) and 2 SD (6.5%) (80).
MM-398
Nanoliposomal irinotecan, also known as PEP02, or MM-398, is a PEGylated liposomal formulation of irinotecan designed to offer higher drug load and stability, as compared to standard irinotecan. This formulation was able to carry a drug load of 109,000 irinotecan molecules per nanoparticle, nearly 10-20 times that of other liposomal formulations (82). In vivo studies showed that MM-398 achieved prolonged circulation compared to standard irinotecan, with 23.2% of injected dose of MM-398 present at 24 hours, versus only 2% of injected dose of standard irinotecan remaining at 30 minutes (82). In preclinical models, MM-398 showed both higher antitumor efficacy and lower toxicity compared to standard irinotecan in models of squamous cell lung cancer (96) and small cell lung cancer (96). In a Phase I dose finding study of 11 patients (including 1 NSCLC), a MTD of 120 mg/m2 IV on Day 1 of a 3 week cycle resulted in 2 PR and 3 SD among ten patients evaluable for response, for a disease control rate of 50% (97). Unfortunately, the one NSCLC patient included in this study was removed from the study after prolonged treatment interruption (due to catheter-based infection). MM-398 was well tolerated with the most common hematologic toxicity being grade I/II anemia and neutropenia, and the most common non-hematologic toxicity being grade I/II diarrhea, nausea and alopecia. There was only one episode of grade III catheter-related infection at the MTD of 120 mg/m2 (97).
Lipoplatin
PEGylated liposomal cisplatin, or LipoplatinTM, was designed by Regulon, Inc. Mountain View, CA, USA and has been investigated in multiple NSCLC studies in Europe. Lipoplatin measures 110 nm in size, and consists of a lipid shell [containing dipalmitoyl phosphatidyl glycerol (DPPG), soy phosphatidyl choline (SPC-3), cholesterol and methoxy-polyethylene glycol-distearoyl phosphatidylethanolamine (mPEG2000-DSPE)], and a cisplatin-containing core (10). The LipoplatinTM formulation is 8.9% cisplatin and 91.1% lipid by weight.
Preclinical studies demonstrated that LipoplatinTM has antitumor efficacy (98), with up to 50 times higher intratumoral concentrations compared to normal tissues (99), and less renal toxicity compared to standard cisplatin (100). A mouse model study of NSCLC showed that LipoplatinTM had superior cytotoxicity in malignant cells but lower toxicity in normal cells, compared to cisplatin (101). This study also found a direct correlation between lipoplatin resistance and excision repair cross-complementing 1 (ERCC1) and lung resistance protein (LRP) expression (101). These two markers are known predictors of response to cisplatin (102-105), and may have similar utility for LipoplatinTM. DNA mismatch repair (MMR) status has also been identified as a predictor of response to lipoplatin, with MLH1-deficient cell lines showing in vitro resistance to lipoplatin (106).
A phase I study of 2nd-line LipoplatinTM with gemcitabine in 13 patients with platinum pretreated, advanced NSCLC found a MTD of LipoplatinTM 120 mg/m2 (107). The overall disease control rate was 23% (three patients), with median OS 29 weeks (range, 4-59 weeks) and median TTP 12 weeks (range, 3-36 weeks). The combination was well tolerated with grade III or higher toxicity (nausea/vomiting, flu-like syndrome) only occurring at dose level 4 (130 mg/m2). A subsequent phase II study of 88 patients with advanced NSCLC compared LipoplatinTM 120 mg/m2 IV on Days 1, 8 + gemcitabine 1,000 mg/m2 IV on Days 1, 8 of a 3 week cycle (n=47) versus cisplatin 100 mg/m2 IV on Day 1 + gemcitabine 1,000 mg/m2 IV on Days 1, 8 of a 3 week cycle (n=44) (108). The ORR for LipoplatinTM and cisplatin were not statistically different (31.7% vs. 25.6%). Of note, the lipoplatin regimen achieved significantly better responses among adenocarcinoma histology subtypes (16.7% PD), compared to squamous histology (46.1% PD). There was less toxicity with LipoplatinTM, particularly less nephrotoxicity (14.6% vs. 25% grades I-IV).
A phase III multicenter study of chemotherapy-naïve, inoperable NSCLC compared LipoplatinTM 200 mg/m2 IV on Day 1 + paclitaxel 135 mg/m2 IV on Day 1 of a 2 week cycle (n=102) versus cisplatin 75 mg/m2 IV on Day 1 + paclitaxel 135 mg/m2 IV on Day 1 of a 2 week cycle (n=100) (109). Grade I-IV nephrotoxicity was significantly lower for LipoplatinTM (6.1% vs. 40%, P<0.001), as were grade I-IV neutropenia (33.3% vs. 45.2%, P=0.017) and grade I-IV nausea/vomiting (32.5% vs. 45.2%, P=0.042). Median OS (9 vs. 10 months) and TTP (6.5 vs. 6 months) were not statistically significantly different. Overall response rates were not statistically significant between the two arms. However, non-squamous histologies showed a better response rate (59.5% vs. 42.5%) and median survival (10 vs. 8 months) after 18 months, with double the number of surviving patients (110).
LipoplatinTM has been studied as monotherapy in NSCLC, at a dose of 200 mg/m2 IV Days 1, 2 of a 2 week cycle (111). Among 21 patients (19 with ≥1 prior chemotherapy), there were 38.1% PR and 42.9% SD. Toxicity included two patients (9.5%) with grade I myelotoxicity, four patients (19.1%) with grade I nausea/vomiting and no nephrotoxicity.
A study was performed to assess the impact of LipoplatinTM on renal insufficiency (112). A total of 40 patients with solid tumors (including 16 NSCLC) who had median serum creatinine 2.4 mg/dL (range, 1.6-3.5 mg/dL) were treated with LipoplatinTM 150-200 mg/m2 IV Day 1 and gemcitabine 1,000 mg/m2 IV Day 1 on a 2 week cycle. Although grade I-II myelotoxicity occurred (attributed to gemcitabine), there were no patients with increased serum creatinine, suggesting that patients with renal insufficiency and NSCLC may be considered eligible for treatment with LipoplatinTM.
Polymeric nanoparticles
Polymer nanoparticle drug delivery systems, are solid, colloidal systems composed of a polymer matrix to which a cytotoxic drug is either covalently attached, dissolved, encapsulated or entrapped within. Polymer nanoparticles may be engineered either using natural polymers, such as albumin, chitosan and heparin, or using synthetic polymers, such as polyethylene glycol (PEG), poly-L-glutamic acid (PGA), polylactic acid (PLA), poly(D,L-lactide-co-glycolic) acid (PLGA), and N-(2-hydroxypropyl)-methacrylamide copolymer (HPMA). One advantage to the use of polymeric nanoparticles is the ability for “controlled release”, whereby the rate of biodegradation and drug diffusion through the polymer matrix can be tuned to achieve controlled release kinetics, with release durations ranging from minutes up to weeks. Conjugation of PLGA to doxorubicin nanoparticles, for example, increased drug release from 5 days for unconjugated doxorubicin up to 25 days for conjugated PLGA-doxorubicin (113). Polymer nanoparticle formulations have been designed for several cytotoxic drugs, including taxanes (8,13,114), camptothecin (115), anthracyclines (116), and topoisomerase II inhibitors (117).
Abraxane
Albumin-bound nanoparticle paclitaxel, also known as nab-paclitaxel, or Abraxane, is FDA approved for 1st line therapy of advanced/metastatic NSCLC, as well as metastatic breast cancer and metastatic pancreatic cancer. In preclinical models, nab-paclitaxel demonstrated higher mean maximum blood concentration, higher intratumoral concentration, greater transport across endothelial cell layers, and higher antitumor activity, when compared to solvent based paclitaxel (118). This was corroborated in human pharmacokinetic studies, which showed higher systemic exposure to free paclitaxel in patients receiving nab-paclitaxel versus those receiving solvent-based paclitaxel (114). A phase III study comparing nab-paclitaxel and carboplatin versus standard solvent based paclitaxel and carboplatin as 1st line therapy for advanced or metastatic NSCLC, found improved antitumor activity and tolerability, leading to FDA approval as 1st line therapy in this setting (8). Patients treated with nab-paclitaxel had significantly less sensory neuropathy, neutropenia, arthralgia and myalgia compared to those receiving solvent based paclitaxel. Subgroup analysis demonstrated that elderly patients (70 years and older) treated with nab-paclitaxel had improved median OS (19.9 vs. 10.4 months, P=0.009) (119).
BIND-014
BIND-014 (BIND Therapeutics, Inc.) is a PEG-PGLA copolymer nanoparticle containing docetaxel that is targeted against PSMA. PSMA is a prostate epithelial cell surface membrane glycoprotein found on the surface of both primary prostate tumor cells and metastatic prostate cancer cells, as well as the neovasculature of other non-prostate solid tumors (120-122). In one study, 5 out of 5 (100%) NSCLC tumor samples showed overexpression of PSMA (120). BIND-014 features an S,S-2-[3-[5-amino-1-carboxypentyl]-ureido]-pentanedioic acid (ACUPA) moiety, which functions as a PSMA substrate analog inhibitor to target PSMA on target cancer cells (13).
In mouse models, BIND-014 showed enhanced tumor accumulation at 12 hours and prolonged tumor growth suppression compared to solvent-based docetaxel (13). Additional pre-clinical and clinical PK studies in both mice and monkeys have shown that BIND-014 achieves higher Cmax and AUC with reduced drug clearance and volume of distribution compared to solvent-based docetaxel (123).
A phase I study of 5 different solid tumors (prostate, cervical, NSCLC, breast and hepatobiliary) showed immunohistochemical detection of PSMA in prostate epithelium and the neovasculature of cervical, anal, NSCLC, and hepatobiliary tumors (124). All patients experienced some objective response to BIND-014 (CR, PR, or SD). A phase I safety and dose-finding study of 30 patients with solid tumors (NSCLC, 6; hepatobiliary, 5; head and neck, 3; prostate, 2; and other solid tumors, 14), resulted in 3 PR (NSCLC, cervical and hepatobiliary), as well as 5 SD (prostate, pancreas, hepatobiliary, head and neck, and anal) (125). BIND-014 was shown to have a dose-linear PK profile with prolonged circulation compared to solvent based docetaxel. The MTD was 60 mg/m2 IV on Day 1 of a 3 week cycle. A separate phase I safety and tolerability study was performed to assess BIND-014 on a weekly dosing schedule (126). A total of 27 patients were evaluable, including three NSCLC. PK studies again showed dose-linear PK and prolonged circulation. There were two confirmed PR (breast, esophagus), and 4 SD >12 weeks (including 1 NSCLC). The MTD was 40 mg/m2 on Days 1, 8, and 15 of a 4-week cycle, resulting in a 50% increase in dose exposure over 28 days compared to 3 week cycle dosing.
A subsequent phase II study treated 40 patients with stage III/IV NSCLC with known genomic status (EGFR, ALK or KRAS), using BIND-014 60 mg/m2 on Day 1 of a 3-week cycle, with ORR as the primary endpoint (127). Among the 40 patients enrolled, a median of 3 doses was administered (range, 1-12). Out of 33 patients evaluable for response, there were 5 (15%) PR and 12 (36%) SD lasting ≥12 weeks. Among 8 patients with KRAS mutations, 2 (25%) had PR and 3 (38%) had SD ≥12 weeks, for a total disease control rate of 63% among KRAS mutants. Grade III/IV hematologic toxicities included anemia (8%) and lymphocytopenia (5%), while grade III/IV non-hematologic toxicity included fatigue (13%), dehydration (10%), peripheral neuropathy (3%), dyspnea (3%), and hypoxia (3%).
There are currently two phase II involving BIND-014 in NSCLC. The first is a phase II to assess safety and efficacy as second-line therapy in NSCLC patients who failed one prior platinum-based regimen for advanced or metastatic disease. The primary outcome will be number of patients with either CR or PR, and the secondary outcome number of patients with adverse events. A second phase II study will assess BIND-014 as second-line therapy for NSCLC patients with KRAS mutations or squamous cell histology. The primary outcome will be disease control rate, and the secondary outcomes will include PFS, OS, duration of response, time to response, and safety and tolerability.
Polymeric micelles
Polymeric micelles utilize self-assembling amphiphilic block copolymers to form a hydrophilic outer shell region that surrounds a hydrophobic core (26). This approach is particularly useful for the delivery of hydrophobic drugs in an aqueous solution, although hydrophilic drugs can also be coupled to the outer hydrophilic surface as well (128). For the hydrophilic segment, many amphiphilic copolymers use PEG, while for the hydrophobic segment, a variety of polyester or poly (amino acid) derivatives may be used, such as PLA or PGA (26). Polymeric micelles have several features which improve their thermodynamic stability, including increased hydrophobic segment length (129), crystallinity (130), cross-linking of the shell or core (131,132), and cohesive forces between drug and core (133,134). In addition to their ability to solubilize drugs in aqueous solution, polymeric micelles are often smaller than 50 nm in size, which allows for prolonged circulation and improved evasion of the RES.
GENEXOL-PM
Genexol-PM, also known as Cynviloq, is a polymeric micelle loaded paclitaxel formulation approved for NSCLC in South Korea. The drug was originally designed by Samyang Co, Seoul, Korea, to avoid the toxicities associated with Cremophor, the lipid based solvent traditionally used to formulate paclitaxel. Genexol-PM consists of a monomethoxy polyethylene glycol-poly (D,L-lactic acid (mPEG-PDLLA) amphiphilic diblock copolymer with a paclitaxel drug load within its hydrophobic core. In pre-clinical models, Genexol-PM displayed higher biodistribution, higher maximum tolerated dose, and higher antitumor efficacy compared to solvent based paclitaxel (14). In vitro studies and mouse xenograft studies have also demonstrated that Genexol-PM may be more effective at radiosensitizing compared to solvent based paclitaxel in NSCLC (135).
A phase I safety and tolerability study among 21 patients with pretreated solid tumors (eight with lung cancer), identified the MTD of 390 mg/m2 IV on Day 1 of a 3 week cycle, with neutropenia, myalgia and neuropathy the main dose-limiting toxicities. The recommended dose was 300 mg/m2 on Day 1 of a 3 week cycle. There were 3 PR (14%), including 2 taxane-pretreated patients (1 NSCLC) and 1 small cell lung cancer (taxane-naïve) (136). In another phase I study, 24 pretreated solid tumor patients (7 lung cancer, 11 taxane-pretreated) were treated with weekly dosing of Genexol-PM, and identified a MTD of 180 mg/m2 IV on Days 1, 8, and 15 of a 4-week cycle. There were 5 PR (21%), including 2 with lung cancer, and 9 SD (38%) (137).
In a multicenter phase II study, 69 patients with treatment naïve advanced NSCLC were treated using a combination of Genexol-PM 230 mg/m2 IV on Day 1 and cisplatin 60 mg/m2 IV on Day 1 of a 3-week cycle (138). Genexol-PM was dose escalated to 300 mg/m2 if no toxicities were observed after the first cycle. Among 69 patients, 77% had stage IV disease, and histology types included adenocarcinoma (58%), squamous (20%), large cell (3%) and other (19%). The ORR was 37.7%; 20 patients (29.0%) achieved SD. The median response duration of 26 responders was 19.8 weeks. Median TTP for all patients was 5.8 months and median OS was 21.7 months with a median follow-up of 9.6 months. Hematologic toxicity included grade III/IV neutropenia (46.4%), febrile neutropenia (3%), and grade III anemia (3%). Non-hematologic toxicity included grade III peripheral neuropathy (13%), grade III/IV myalgia (5.8%), and grade III/IV arthralgia (7.3%). There was no grade IV neuropathy. Dose reductions were required for 7 patients (10%) due to toxicity.
In another multicenter phase IIB study, the combination of Genexol-PM with cisplatin was directly compared against solvent-based paclitaxel with cisplatin (139). A total of 276 patients with advanced NSCLC were randomized to either Genexol-PM 230 mg/m2 IV on Day 1 and cisplatin 60 mg/m2 IV on Day 1 of a 3-week cycle (n=140) versus solvent-based paclitaxel 175 mg/m2 IV on Day 1 and cisplatin 60 mg/m2 IV on Day 1 of a 3-week cycle (n=136). Histology subtypes included adenocarcinoma (50.4%), squamous cell (40.2%), large cell (1.8%) and other (7.6%). ORR for the Genexol-PM arm was 44%, compared to 42% for the solvent-based paclitaxel arm. Median PFS (5.4 vs. 5.5 months), median OS (15.1 vs. 14.0 months) and 1-year survival rates (62% vs. 55%) were not significantly different. Incidence of grade III/IV toxicity was similar between both arms. Grade III/IV neutropenia was higher in the Genexol-PM arm (P=0.04), but there was no significant increase in febrile neutropenia (P=0.8). As such, this study demonstrated noninferiority for Genexol-PM with cisplatin compared to standard solvent-based paclitaxel with cisplatin in NSCLC.
Genexol-PM was studied in combination with gemcitabine in a phase II study for advanced NSCLC (140). A total of 43 patients were treated with Genexol-PM at 230 mg/m2 IV on Day 1 and gemcitabine 1,000 mg/m2 IV on Day 1 and Day 8 of a 3-week cycle. Histology types included adenocarcinoma (65%), squamous (19%), and other (16%). Twenty-three patients (53%) were EGFR wild type, 3 patients (7%) EGFR mutant, and 17 patients (40%) were unknown. The ORR was 46.5%, with 0 CR and 20 PR. Median PFS was 4.0 months, and median OS was 14.8 months. Grade III/IV toxicity occurred in 22 patients (51.2%). Grade III/IV hematologic toxicity included neutropenia (16%) and neutropenic fever (9%). The most common grade III/IV non-hematologic toxicities were pneumonia (12%), asthenia (7%), pulmonary thromboembolism (7%), myalgia (5%), peripheral neuropathy (5%), diarrhea (2%), skin rash (2%), and dyspnea (5%).
Preliminary results from a phase II study combining Genexol-PM with carboplatin were presented at the ASCO 2014 Annual Meeting (141). A total of 80 patients with stage IIIB/IV NSCLC were treated with Genexol-PM 230 mg/m2 IV on Day 1 and Carboplatin AUC 6 IV on Day 1 of a 3-week cycle for a maximum of 6 cycles. Clinical responses included 40.7% PR, and 48.2% SD. Hematologic toxicity was manageable, varying from grade I-III, and included 22 patients (27.5%) with grade III neutropenia.
NC-6004
NC-6004 is a 28 nm diameter, polymer-metal micellar nanoparticle composed of a hydrophilic PEG outer shell and an inner core that contains a coordinate complex of polyglutamate [P(Glu)] with cisplatin (15). In comparison to other cisplatin-containing polymeric micelles, this formulation was able to achieve a relatively much higher drug composition of 39% by weight. NC-6004 is extremely stable in distilled water with prolonged decay, and even under diluted conditions, showed a sustained release pattern >150 hours with no initial burst of drug release. NC-6004 showed prolonged plasma circulation, increased intratumoral accumulation and decreased accumulation in normal organs, when compared to standard free cisplatin in mouse models. In vivo antitumor activity of NC-6004 was demonstrated in mouse models of both lung and colon cancer, and interestingly, the investigators were also able to achieve complete tumor regression in 6 out of 10 mice with colon adenocarcinoma.
In a preclinical study using rodent models, NC-6004 showed decreased clearance and longer circulation time compared to standard cisplatin (142). NC-6004 achieved an AUC0-t 65 times that of cisplatin (P<0.001) and a Cmax 8 times that of cisplatin (P=0.001). Meanwhile, the CLtot of NC-6004 was one-nineteenth of that of cisplatin (P<0.01). NC-6004 also showed greater tumor accumulation compared to cisplatin. Peak tumor platinum concentrations for NC-6004 occurred at 48 hours after administration, compared to 10 minutes after administration for cisplatin. Intratumoral maximum concentration (Cmax) was 2.5 times higher for NC-6004 than cisplatin (P<0.001), and intratumoral AUC was 3.6 times higher for NC-6004 than cisplatin. For gastric cancer implanted mice, NC-6004 showed antitumor activity equivalent to or greater than cisplatin. Meanwhile, NC-6004 resulted in significantly less neuropathy and nephrotoxicity compared to cisplatin. A separate preclinical study also found that NC 6004 resulted in decreased ototoxicity among guinea pigs, with decreased platinum distribution and lower platinum concentration in the organ of Corti (P<0.01), when compared to standard cisplatin (143). NC-6004 has also been found to be effective in mouse models of oxaliplatin-resistant tumors (144).
Based on these preclinical studies, an open-label, dose-escalating phase I study of NC-6004 was conducted among 17 patients with advanced solid tumors in the UK (145). Tumor types included: colon [4], lung [3], esophagus [2], melanoma [2], pancreas [2], GIST [1], renal [1], mesothelioma [1], and hepatobiliary [1]. Patients were treated with NC-6004 IV on Day 1 of a 3 week cycle, with the MTD identified as 120 mg/m2 IV on Day 1 of a 3-week cycle and the recommended dose 90 mg/m2 IV on Day 1 of a 3-week cycle. The maximum number of cycles received was four cycles (three patients), and mean number of cycles was 2.4. Pharmacokinetic data showed that maximum plasma concentration and AUC of ultra-filterable platinum after NC-6004 were 1/34 and 8.5 folds of those with free cisplatin. NC-6004 was well tolerated from a hematologic toxicity standpoint, with only one episode of grade III thrombocytopenia at 10 mg/m2 and one patient with grade I thrombocytopenia at 90 mg/m2. Regarding non-hematologic toxicity, the most common adverse events were fatigue (52.9%), nausea (47.1%), vomiting (42.1%), and renal impairment (35.3%). There was no observed ototoxicity or neuropathy at any dose level. SD was observed in seven patients (41.2%) for longer than 4 weeks, including two patients with lung cancers. At dose levels 10-60 mg/m2, only 2 out of 8 patients achieved SD (25%), while at doses 90-120 mg/m2, 50% and 67% achieved SD, suggesting higher efficacy with higher doses of NC-6004. Overall, median PFS was 49 days.
A phase Ib/II dose escalation and expansion study of NC-6004 is currently enrolling patients with advanced stage IIIB/IV squamous and non-squamous NSCLC on second- or third-line therapy, as well as other patients with advanced solid tumors, to be treated with the combination of NC-6004 and gemcitabine. The primary outcomes will be maximum tolerated dose of NC-6004, and the secondary outcomes will assess overall response rate, based on CR and PR.
Conclusions
The recent introduction of immunotherapy has made a significant impact in the treatment of lung cancer, with improved outcomes including higher response rates, PFS and OS. KEYNOTE-001 treated 495 patients with advanced NSCLC using pembrolizumab, an anti-PD-1 Ab, in advanced NSCLC; the objective RR among all patients was 19.4%, with median duration of response 12.5 months, median duration of PFS 3.7 months and median duration of OS 12.0 months (7). In CheckMate 017, a total of 272 patients with advanced pre-treated squamous cell NSCLC were randomized to either anti-PD-1 therapy with nivolumab or docetaxel. Compared to docetaxel, nivolumab produced higher median OS (9.2 vs. 6.0 months), and higher response rates (20% vs. 9%). Median PFS was 3.5 months for patients treated with nivolumab (6).
While both of these studies demonstrated the utility of immunotherapy alone in NSCLC, it is unclear how to use immunotherapy in combination with chemotherapy. A phase II study randomized 204 patients with chemotherapy naïve, advanced NSCLC to treatment with carboplatin and paclitaxel plus either “concurrent” ipilimumab (anti-CTLA-4 Ab), “phased” ipilimumab, or placebo (5). “Concurrent” dosing consisted of ipilimumab given up front with the first four cycles of chemotherapy, while “phased” dosing required that the first two cycles of chemotherapy be given alone, and then ipilimumab was added for subsequent cycles. Though this study suggested a PFS benefit from “phased” dosing of ipilimumab, there is currently no standard approach to the use of chemotherapy with immunotherapy for NSCLC. More importantly, for the many patients who do not respond to immunotherapy, chemotherapy may be their only remaining treatment option. Consequently, the need to improve chemotherapy remains as vital as ever.
Targeted therapy has improved treatment of NSCLC compared to chemotherapy as well (3,4), but is also a strategy that has its limitations. EGFR mutations only occur in approximately 10-20% of North American or European populations with lung adenocarcinomas, although this number may be as high as 60% in Asian populations (146). ALK and ROS1 mutations are much less common, and occur in approximately 5% and 1% of lung adenocarcinoma, respectively (146). In addition to patients who do not have these targetable mutations, there are also patients who have mutations but develop resistance to target inhibitors. Resistance to EGFR inhibitors has been attributed to secondary EGFR mutations (T790M) in up to 60% of patients (147); other less frequent mechanisms for EGFR resistance include HER2 amplification, c-MET amplification, and PI3KCA mutations (148). In contrast, resistance to ALK inhibitors occurs due to secondary mutations in only 1/3 of cases, with other mechanisms including EGFR co-activation, KIT amplification, KRAS activation or IGF-1 receptor activation (149). For patients who develop resistance to targeted therapy, chemotherapy remains the standard for restoring responsiveness.
Chemotherapy for NSCLC has not significantly been improved since the 1990s. Systemic toxicity associated with platinum doublet chemotherapy regimens presents one of the key challenges in this arena. Nanoparticle therapies present a new arsenal of effective, but less toxic anticancer agents to fit this need. Through their unique size, nanoparticles employ the EPR effect, resulting in increased intratumoral concentrations and decreased systemic toxicity to normal tissues. Clinically these formulations demonstrate significantly better tolerability compared to standard chemotherapy formulations, including: (I) decreased cardiotoxicity for nanoparticle anthracyclines; (II) less neuropathy and renal toxicity for nanoparticle platinums; and (III) less neuropathy and myelosuppression for nanoparticle taxanes. Due to their unique size dependent properties, nanotherapies are able to carry higher amounts of cytotoxic drug, bind to multivalent ligands for improved tumor targeting, and overcome membrane pump drug resistance mechanisms, all which may contribute to higher antitumor efficacy as well. As nanotechnology improves, this should inevitably result in a growing number of new anticancer agents, each with their own unique advantages.
Acknowledgments
The authors would like to acknowledge Yekaterina Kadyshevskaya and Peter Kuhn for their assistance with illustrations.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyudmila Bazhenova and Ajay Pal Singh Sandhu) for the series “Recent advances in radiotherapy and targeted therapies for lung cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.08.05). The series “Recent advances in radiotherapy and targeted therapies for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma . Cancer 2001;92:2639-47. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [PubMed]
- Langer CJ, Hirsh V, Okamoto I, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer 2015;113:20-9. [PubMed]
- Stathopoulos GP, Boulikas T. Lipoplatin formulation review article. J Drug Deliv 2012;2012:581363.
- Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release 2012;160:117-34. [PubMed]
- Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother 2006;7:1041-53. [PubMed]
- Hrkach J, Von Hoff D, Mukkaram Ali M, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 2012;4:128ra39.
- Kim SC, Kim DW, Shim YH, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release 2001;72:191-202. [PubMed]
- Nishiyama N, Okazaki S, Cabral H, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 2003;63:8977-83. [PubMed]
- NNI. What is nanotechnology? Avaliable online: http://www.nano.gov/
- TRS. Nanoscience and nanotechnologies: opportunities and uncertainties. 2004. Avaliable online: https://royalsociety.org/policy/publications/2004/nanoscience-nanotechnologies/
- Rogers-Nieman GM, Dinu CZ. Therapeutic applications of carbon nanotubes: opportunities and challenges. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014;6:327-37. [PubMed]
- Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines 2010;9:1095-107. [PubMed]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003;2:347-60. [PubMed]
- Cascone MG, Lazzeri L, Carmignani C, et al. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mater Sci Mater Med 2002;13:523-6. [PubMed]
- Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm 2004;284:109-22. [PubMed]
- Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 2008;14:1310-6. [PubMed]
- Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med 2008;59:251-65. [PubMed]
- Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165-70. [PubMed]
- Gaucher G, Dufresne MH, Sant VP, et al. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release 2005;109:169-88. [PubMed]
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 1999;17:593-623. [PubMed]
- Wisse E, Braet F, Luo D, et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol 1996;24:100-11. [PubMed]
- Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst 1989;6:193-210. [PubMed]
- Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 1995;55:3752-6. [PubMed]
- Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release 2012;164:138-44. [PubMed]
- Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev 2003;55:403-19. [PubMed]
- Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res 2003;42:463-78. [PubMed]
- Bae YH, Huh KM, Kim Y, et al. Biodegradable amphiphilic multiblock copolymers and their implications for biomedical applications. J Control Release 2000;64:3-13. [PubMed]
- Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A 2008;105:11613-8. [PubMed]
- Decuzzi P, Pasqualini R, Arap W, et al. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res 2009;26:235-43. [PubMed]
- Gentile F, Chiappini C, Fine D, et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech 2008;41:2312-8. [PubMed]
- Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2007;2:249-55. [PubMed]
- Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 2002;115:849-56. [PubMed]
- Wu J, Akaike T, Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res 1998;58:159-65. [PubMed]
- Wu J, Akaike T, Hayashida K, et al. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res 2001;92:439-51. [PubMed]
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006;11:812-8. [PubMed]
- Seymour LW, Miyamoto Y, Maeda H, et al. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur J Cancer 1995;31A:766-70. [PubMed]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46:6387-92. [PubMed]
- Ko J, Park K, Kim YS, et al. Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(beta-amino ester) block copolymer micelles for cancer therapy. J Control Release 2007;123:109-15. [PubMed]
- Yatvin MB, Kreutz W, Horwitz BA, et al. pH-sensitive liposomes: possible clinical implications. Science 1980;210:1253-5. [PubMed]
- Pelicano H, Martin DS, Xu RH, et al. Glycolysis inhibition for anticancer treatment. Oncogene 2006;25:4633-46. [PubMed]
- Ab O, Whiteman KR, Bartle LM, et al. IMGN853, a Folate Receptor-
α (FRα )-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRα -Expressing Tumors. Mol Cancer Ther 2015;14:1605-13. [PubMed] - Wang XB, Zhou HY. Molecularly targeted gemcitabine-loaded nanoparticulate system towards the treatment of EGFR overexpressing lung cancer. Biomed Pharmacother 2015;70:123-8. [PubMed]
- Maya S, Sarmento B, Lakshmanan VK, et al. Chitosan cross-linked docetaxel loaded EGF receptor targeted nanoparticles for lung cancer cells. Int J Biol Macromol 2014;69:532-41. [PubMed]
- Guo Y, Wang L, Lv P, et al. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett 2015;9:1065-72. [PubMed]
- Alibolandi M, Ramezani M, Abnous K, et al. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release 2015;209:88-100. [PubMed]
- Chi L, Na MH, Jung HK, et al. Enhanced delivery of liposomes to lung tumor through targeting interleukin-4 receptor on both tumor cells and tumor endothelial cells. J Control Release 2015;209:327-36. [PubMed]
- Zhang Y, Zhang X, Liu H, et al. Mixed nanomicelles as potential carriers for systemic delivery of Z-GP-Dox, an FAP
α -based doxorubicin prodrug: formulation and pharmacokinetic evaluation. Int J Nanomedicine 2015;10:1625-36. [PubMed] - Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006;66:6732-40. [PubMed]
- Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999;39:361-98. [PubMed]
- Ling V, Thompson LH. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol 1974;83:103-16. [PubMed]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976;455:152-62. [PubMed]
- Ueda K, Cardarelli C, Gottesman MM, et al. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A 1987;84:3004-8. [PubMed]
- Slovak ML, Hoeltge GA, Dalton WS, et al. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res 1988;48:2793-7. [PubMed]
- Horio M, Gottesman MM, Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A 1988;85:3580-4. [PubMed]
- Wils P, Phung-Ba V, Warnery A, et al. Polarized transport of docetaxel and vinblastine mediated by P-glycoprotein in human intestinal epithelial cell monolayers. Biochem Pharmacol 1994;48:1528-30. [PubMed]
- Sugiyama Y, Kato Y, Chu X. Multiplicity of biliary excretion mechanisms for the camptothecin derivative irinotecan (CPT-11), its metabolite SN-38, and its glucuronide: role of canalicular multispecific organic anion transporter and P-glycoprotein. Cancer Chemother Pharmacol 1998;42:S44-9. [PubMed]
- Shen H, Lee FY, Gan J. Ixabepilone, a novel microtubule-targeting agent for breast cancer, is a substrate for P-glycoprotein (P-gp/MDR1/ABCB1) but not breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther 2011;337:423-32. [PubMed]
- Young LC, Campling BG, Voskoglou-Nomikos T, et al. Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res 1999;5:673-80. [PubMed]
- Oka M, Fukuda M, Sakamoto A, et al. The clinical role of MDR1 gene expression in human lung cancer. Anticancer Res 1997;17:721-4. [PubMed]
- Zaman GJ, Flens MJ, van Leusden MR, et al. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A 1994;91:8822-6. [PubMed]
- Lutzky J, Astor MB, Taub RN, et al. Role of glutathione and dependent enzymes in anthracycline-resistant HL60/AR cells. Cancer Res 1989;49:4120-5. [PubMed]
- Zeng H, Chen ZS, Belinsky MG, et al. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res 2001;61:7225-32. [PubMed]
- Loe DW, Deeley RG, Cole SP. Characterization of vincristine transport by the M(r) 190,000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res 1998;58:5130-6. [PubMed]
- Schneider E, Yamazaki H, Sinha BK, et al. Buthionine sulphoximine-mediated sensitisation of etoposide-resistant human breast cancer MCF7 cells overexpressing the multidrug resistance-associated protein involves increased drug accumulation. Br J Cancer 1995;71:738-43. [PubMed]
- Barnouin K, Leier I, Jedlitschky G, et al. Multidrug resistance protein-mediated transport of chlorambucil and melphalan conjugated to glutathione. Br J Cancer 1998;77:201-9. [PubMed]
- Nooter K, Bosman FT, Burger H, et al. Expression of the multidrug resistance-associated protein (MRP) gene in primary non-small-cell lung cancer. Ann Oncol 1996;7:75-81. [PubMed]
- Kunjachan S, Rychlik B, Storm G, et al. Multidrug resistance: Physiological principles and nanomedical solutions. Adv Drug Deliv Rev 2013;65:1852-65. [PubMed]
- Lv S, Tang Z, Li M, et al. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 2014;35:6118-29. [PubMed]
- Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 2012;17:1044-52. [PubMed]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 2005;4:145-60. [PubMed]
- Numico G, Castiglione F, Granetto C, et al. Single-agent pegylated liposomal doxorubicin (Caelix) in chemotherapy pretreated non-small cell lung cancer patients: a pilot trial. Lung Cancer 2002;35:59-64. [PubMed]
- Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol 1999;17:3512-21. [PubMed]
- Xenidis N, Vardakis N, Varthalitis I, et al. A multicenter phase II study of pegylated liposomal doxorubicin in combination with irinotecan as second-line treatment of patients with refractory small-cell lung cancer. Cancer Chemother Pharmacol 2011;68:63-8. [PubMed]
- Stathopoulos GP, Boulikas T, Vougiouka M, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep 2005;13:589-95. [PubMed]
- Drummond DC, Noble CO, Guo Z, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 2006;66:3271-7. [PubMed]
- Hagemeister F, Rodriguez MA, Deitcher SR, et al. Long term results of a phase 2 study of vincristine sulfate liposome injection (Marqibo(®)) substituted for non-liposomal vincristine in cyclophosphamide, doxorubicin, vincristine, prednisone with or without rituximab for patients with untreated aggressive non-Hodgkin lymphomas. Br J Haematol 2013;162:631-8. [PubMed]
- Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5:3394-402. [PubMed]
- Bomgaars L, Geyer JR, Franklin J, et al. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol 2004;22:3916-21. [PubMed]
- Rueda Domínguez A, Olmos Hidalgo D, Viciana Garrido R, et al. Liposomal cytarabine (DepoCyte) for the treatment of neoplastic meningitis. Clin Transl Oncol 2005;7:232-8. [PubMed]
- O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440-9. [PubMed]
- Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 2000;11:1029-33. [PubMed]
- Gill SE, Savage K, Wysham WZ, et al. Continuing routine cardiac surveillance in long-term use of pegylated liposomal doxorubicin: is it necessary? Gynecol Oncol 2013;129:544-7. [PubMed]
- Patlakas G, Bouros D, Tsantekidou-Pozova S, et al. Triplet chemotherapy with docetaxel, gemcitabine and liposomal doxorubicin, supported with subcutaneous amifostine and hemopoietic growth factors, in advanced non-small cell lung cancer. Anticancer Res 2005;25:1427-31. [PubMed]
- Koukourakis MI, Romanidis K, Froudarakis M, et al. Concurrent administration of Docetaxel and Stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer 2002;87:385-92. [PubMed]
- Varveris H, Kachris S, Mazonakis M, et al. Pegulated liposomal doxorubicin and cisplatin given concurrently with conventional radiotherapy: a phase I dose-escalation trial for patients with squamous cell carcinoma of head and neck and lung. Oncol Rep 2004;12:473-81. [PubMed]
- Tsoutsou PG, Froudarakis ME, Bouros D, et al. Hypofractionated/accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res 2008;28:1349-54. [PubMed]
- Samantas E, Kalofonos H, Linardou H, et al. Phase II study of pegylated liposomal doxorubicin: inactive in recurrent small-cell lung cancer. A Hellenic Cooperative Oncology Group Study. Ann Oncol 2000;11:1395-7. [PubMed]
- Leighl NB, Goss GD, Lopez PG, et al. Phase II study of pegylated liposomal doxorubicin HCl (Caelyx) in combination with cyclophosphamide and vincristine as second-line treatment of patients with small cell lung cancer. Lung Cancer 2006;52:327-32. [PubMed]
- Chan Daniel C, Kalra Ashish, Zhang Zhiyong, et al. Abstract 4626: Evaluating the pharmacodynamics and pharmacokinetic effects of MM-398, a nanoliposomal irinotecan (nal-IRI) in subcutaneous xenograft tumor models of human squamous cell carcinoma and small cell lung cancers. Cancer Res 2014;74:4626.
- Chang TC, Shiah HS, Yang CH, et al. Phase I study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients. Cancer Chemother Pharmacol 2015;75:579-86. [PubMed]
- Boulikas T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep 2004;12:3-12. [PubMed]
- Boulikas T, Stathopoulos GP, Volakakis N, et al. Systemic Lipoplatin infusion results in preferential tumor uptake in human studies. Anticancer Res 2005;25:3031-9. [PubMed]
- Devarajan P, Tarabishi R, Mishra J, et al. Low renal toxicity of lipoplatin compared to cisplatin in animals. Anticancer Res 2004;24:2193-200. [PubMed]
- Arienti C, Tesei A, Ravaioli A, et al. Activity of lipoplatin in tumor and in normal cells in vitro. Anticancer Drugs 2008;19:983-90. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Li XQ, Li J, Shi SB, et al. Expression of MRP1, BCRP, LRP and ERCC1 as prognostic factors in non-small cell lung cancer patients receiving postoperative cisplatin-based chemotherapy. Int J Biol Markers 2009;24:230-7. [PubMed]
- Li J, Li ZN, Du YJ, et al. Expression of MRP1, BCRP, LRP, and ERCC1 in advanced non-small-cell lung cancer: correlation with response to chemotherapy and survival. Clin Lung Cancer 2009;10:414-21. [PubMed]
- Li J, Li ZN, Yu LC, et al. Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer 2010;69:116-22. [PubMed]
- Fedier A, Poyet C, Perucchini D, et al. MLH1-deficient tumor cells are resistant to lipoplatin, but retain sensitivity to lipoxal. Anticancer Drugs 2006;17:315-23. [PubMed]
- Froudarakis ME, Pataka A, Pappas P, et al. Phase 1 trial of lipoplatin and gemcitabine as a second-line chemotherapy in patients with nonsmall cell lung carcinoma. Cancer 2008;113:2752-60. [PubMed]
- Mylonakis N, Athanasiou A, Ziras N, et al. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer 2010;68:240-7. [PubMed]
- Stathopoulos GP, Antoniou D, Dimitroulis J, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol 2010;21:2227-32. [PubMed]
- Stathopoulos GP, Antoniou D, Dimitroulis J, et al. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother Pharmacol 2011;68:945-50. [PubMed]
- Stathopoulos GP, Stathopoulos J, Dimitroulis J. Two consecutive days of treatment with liposomal cisplatin in non-small cell lung cancer. Oncol Lett 2012;4:1013-1016. [PubMed]
- Stathopoulos G, Rigatos S, Stathopoulos J, et al. Liposomal cisplatin in cancer patients with renal failure. J Clin Oncol 2011;29.
- Yoo HS, Oh JE, Lee KH, et al. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res 1999;16:1114-8. [PubMed]
- Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 2008;14:4200-5. [PubMed]
- Svenson S, Wolfgang M, Hwang J, et al. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release 2011;153:49-55. [PubMed]
- Vasey PA, Kaye SB, Morrison R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res 1999;5:83-94. [PubMed]
- Yadav KS, Chuttani K, Mishra AK, et al. Effect of Size on the Biodistribution and Blood Clearance of Etoposide-Loaded PLGA Nanoparticles. PDA J Pharm Sci Technol 2011;65:131-9. [PubMed]
- Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006;12:1317-24. [PubMed]
- Socinski MA, Langer CJ, Okamoto I, et al. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:314-21. [PubMed]
- Chang SS, Reuter VE, Heston WD, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 1999;59:3192-8. [PubMed]
- Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81-5. [PubMed]
- Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997;57:3629-34. [PubMed]
- Summa J, Von Hoff D, Sachdev J, et al. Abstract 5514: Pharmacokinetics of BIND-014 (docetaxel nanoparticles for injectable suspension) in preclinical species and patients with advanced solid tumors. AACR 106th Annual Meeting 2015, Philadelphia, USA, 2015.
- Low S, Daniel Von Hoff D, Mita M, et al. Abstract 911: Prostate-specific membrane antigen (PSMA) expression as a potential patient selection marker in patients with refractory solid tumors administered BIND-014, a PSMA-targeted nanoparticle containing docetaxel. AACR Annual Meeting 2014, San Diego, CA, USA, 2014.
- Von Hoff DD, Mita M, Eisenberg P, et al. A Phase 1study of BIND-014, a PSMA-targeted nanoparticle containing docetaxel, in patients with refractory solid tumors; Abstract LB-203 presented at the annual meeting of the American Association for Cancer Research, Washington, DC, USA, 2013.
- Mita M, Burris H, LoRusso P, et al. A phase 1 study of BIND-014, a PSMA-targeted nanoparticle containing docetaxel, administered to patients with refractory solid tumors on a weekly schedule. AACR Annual Meeting 2014, San Diego, CA, USA, 2014:74:CT210.
- Natale R, Socinski M, Hart L, et al. Clinical activity of BIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy in patients (pts) with Stage III/IV non-small cell lung cancer. Eur J Cancer 2014;50:19.
- Bawarski WE, Chidlowsky E, Bharali DJ, et al. Emerging nanopharmaceuticals. Nanomedicine. Nanomedicine 2008;4:273-82. [PubMed]
- Jette KK, Law D, Schmitt EA, et al. Preparation and drug loading of poly(ethylene glycol)-block-poly(epsilon-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharm Res 2004;21:1184-91. [PubMed]
- Slager J, Domb AJ. Biopolymer stereocomplexes. Adv Drug Deliv Rev 2003;55:549-83. [PubMed]
- Shuai X, Merdan T, Schaper AK, et al. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug Chem 2004;15:441-8. [PubMed]
- Jaturanpinyo M, Harada A, Yuan X, et al. Preparation of bionanoreactor based on core-shell structured polyion complex micelles entrapping trypsin in the core cross-linked with glutaraldehyde. Bioconjug Chem 2004;15:344-8. [PubMed]
- Lee J, Cho EC, Cho K. Incorporation and release behavior of hydrophobic drug in functionalized poly(D,L-lactide)-block-poly(ethylene oxide) micelles. J Control Release 2004;94:323-35. [PubMed]
- Li Y, Kwon GS. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl-L-aspartamide). Part I: Effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm Res 2000;17:607-11. [PubMed]
- Werner ME, Cummings ND, Sethi M, et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:463-8. [PubMed]
- Kim TY, Kim DW, Chung JY, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 2004;10:3708-16. [PubMed]
- Lim WT, Tan EH, Toh CK, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol 2010;21:382-8. [PubMed]
- Kim DW, Kim SY, Kim HK, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol 2007;18:2009-14. [PubMed]
- Lee SY, Park HS, Lee KY, et al. Paclitaxel-loaded polymeric micelle (230 mg/m(2)) and cisplatin (60 mg/m(2)) vs. paclitaxel (175 mg/m(2)) and cisplatin (60 mg/m(2)) in advanced non-small-cell lung cancer: a multicenter randomized phase IIB trial. Clin Lung Cancer 2013;14:275-82. [PubMed]
- Ahn HK, Jung M, Sym SJ, et al. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2014;74:277-82. [PubMed]
- Hoa HL. Evaluating efficacy and safety of paclitaxel polymeric micelle regimen in advanced and metastatic NSCLC patients in Hai Phong Oncology Centre Vietnam. J Clin Oncol 2014;32.
- Uchino H, Matsumura Y, Negishi T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer 2005;93:678-87. [PubMed]
- Baba M, Matsumoto Y, Kashio A, et al. Micellization of cisplatin (NC-6004) reduces its ototoxicity in guinea pigs. J Control Release 2012;157:112-7. [PubMed]
- Alami N, Banerjee K, Juste S, et al. NC-6004, a novel cisplatin-incorporated polymeric micelle, is highly effective against oxaliplatin-resistant tumor models. Proc Amer Assoc Cancer Res 2006;47:133.
- Plummer R, Wilson RH, Calvert H, et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br J Cancer 2011;104:593-8. [PubMed]
- Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011;6:e28204 [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17.


