
Bruton’s tyrosine kinase inhibitors in primary central nervous system lymphoma—evaluation of anti-tumor efficacy and brain distribution
Introduction
Primary central nervous system lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma confined to brain, spine, cerebrospinal fluid (CSF) and eyes, without evidence of systemic spread (1). The prognosis of PCNSL has improved during the past decades owing to the application of high-dose methotrexate (HD-MTX), which is now the backbone of first-line treatments for PCNSL patients (2). However, patients treated with MTX and rituximab-based regimens still suffer from frequent recurrence and disease progression (3). Also, current first-line treatments are sometimes unfitted for elderly or certain patients due to systemic toxicity and severe neurotoxicity, which calls for the use of other interventions like targeted medicine with less toxicity (3-5).
Approximately 80–90% of PCNSL cases are diffuse-large B-cell lymphomas (DLBCL) (2), which is associated with unfavorable outcomes (6). In PCNSL and DLBCL, B-cell receptor pathway and MYD88 frequently mutate, leading to constant activation of oncogenic nuclear factor-kappa B (NF-κB) pathway (6). Bruton’s tyrosine kinase (BTK) is a crucial regulator of B-cell receptor pathway and a promising target in treating lymphomas with constitutive NF-κB pathway activation (7). Pharmaceutical inhibition of BTK by BTK inhibitor ibrutinib has already demonstrated promising efficiency in DLBCL (8). As for PCNSL, a preclinical study on ibrutinib reported high level of drug distribution in brain, suggesting possible application of ibrutinib in brain confined lymphomas (9). In two clinical trials, recurrent PCNSL patients who received ibrutinib as single drug achieved objective response rate (ORR) of 50–70% (10,11). Based on the inspiring results of these studies, it is worth exploring BTK inhibitor-based therapy in the treatment of PCNSL.
After the discovery of ibrutinib, second-generation’s BTK inhibitors including zanubrutinib and tirabrutinib were developed, demonstrating greater selectivity and inhibiting activity, better pharmacokinetic properties, broader safety window and less toxicities (12-16). Owing to these qualities, it can be deducted that zanubrutinib and tirabrutinib might also be good anti-PCNSL candidates. Therefore, it is vital to determine the anti-tumor effects of these BTK inhibitors, as well as their ability to penetrate through BBB and distribute in brain.
In present work, we retrospectively reviewed the patients with PCNSL receiving ibrutinib treatment in our hospital. Given the good clinical response of these patients, we further sought to determine and compare the anti-PCNSL ability of three BTK inhibitors, ibrutinib, zanubrutinib and tirabrutinib, by conducting in vitro inhibition and apoptosis assays on DLBCL cells and evaluating their ability to distribute in brain parenchyma in SD rats.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tcr-21-50).
Methods
Patients and specimen collection
A retrospective review was performed on all PCNSL patients who received ibrutinib between August 2017 to May 2020. All lymphomas were histologically confirmed as DLBCL and were located in brain, spine, eyes or CSF without lesions outside the central nervous system (CNS) at the time of diagnosis. Three patients who achieved good response after receiving ibrutinib therapy were included. None of them were using CYP3A4 inhibitors or inducers or other drugs that might alter the bioavailability of ibrutinib. Two hours after ibrutinib therapy, CSF of one patient was obtained by lumbar puncture. CSF sample was immediately centrifuged at 300 g for 10 min (Eppendorf centrifuge 5702R) to remove cellular components and stored in liquid nitrogen for further analysis. Clinical and follow-up data of patients were recorded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients gave written informed consent to participate in the study. This study was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-3).
In vitro study
Cell culture
DLBCL cell lines SU-DHL-6 and OCI-LY10 were obtained from American Type Culture Collection (ATCC, USA). Cells were incubated in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin and 100 µg/mL streptomycin (Gibco, Life Technologies, NY, USA). Cells were incubated at 37 °C in 5% CO2.
Inhibition assay
Cells were collected at logarithmic phase, resuspended and incubated in 96-well plate for 24 hours. Cells were then divided into four groups which were incubated with or without one of the six or ten concentration of ibrutinib, zanubrutinib or tirabrutinib respectively for 72 hours. DMSO was used as control. After incubation, 10 µL Cell Counting Kit-8 (MedChemExpress, NJ, USA) was added to each well and incubated at 37 °C for 2–4 hours to detect cell viability. The optical density values were then measured at 450 nm using SpectraMax i3X Reader (Molecular Devices, CA, USA). Each experiment was repeated twice.
Apoptosis assay
SU-DHL-6cells were seeded (4×105/mL) and incubated in 96-well plate for 24 hours. One day after inoculation, cells were then divided into eight groups which were incubated with or without one of the three concentration of ibrutinib, zanubrutinib or tirabrutinib respectively for 24 or 48 hours. DMSO was used as control. Cellular apoptosis assay was performed by Annexin V and 7-aminoactinomycin D (7-AAD) staining using an Annexin V/7-AAD Apoptosis kit according to the manufacturer’s protocol (eBioscience, 88-8102-72). Following staining, cells were analyzed using flow cytometry (BD FACSCalibur™ platform, BD Biosciences, NJ, USA). Living cells (Annexin V-/7-AAD-), cells undergone early apoptosis (Annexin V+/7-AAD-), cells undergone late apoptosis or dead cells (Annexin V+/7-AAD+) were subsequently identified and counted.
In vivo study
Animals and sample collection
Fifty-four male SD rats weighing 219.0–260.5g were purchased from Zhejiang Vital River Labomouseory Animal Technology Co., Ltd. They were housed in cages with sterilized rodent diet ad libitum, except during overnight fasting prior to dosing and until 4 hours post-dose. Studies involving animals were approved by Ethics Committee for Experimental Animals of Zhejiang Cancer Hospital (2020-12-004). All animals were handled in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China.
SD rats were randomly divided into 3 groups and treated with ibrutinib, zanubrutinib and tirabrutinib, respectively. Eighteen rats in each group were then divided into six subgroups (n=3, per designated time points). 10 mg/kg tirabrutinib, 15 mg/kg zanubrutinib or 55 mg/kg ibrutinib were administered once orally. The dosage of drugs was calculated based on clinical dosage and equivalent surface area dosage conversion factors. Blood samples were collected at six designated time points (0.25, 0.5, 1, 2, 4 and 24 h) after drug administration. 200 µL of blood samples (yielding ~100 µL of plasma) were obtained from the jugular vein of animals and collected in micro K2EDTA tubes. Blood sample were then put on ice and centrifuged at 8,000 rpm for 5 min at 4 °C to get rat plasma sample within 30 minutes of collection. For brain samples, the rats were sacrificed via decapitation at designated time points after drug administration. The skin and muscle were removed to expose the cranium. Cuts were made between the eyes and at interparietal bone for the exposure and removal of brain. Once obtained, the brain samples were added with 3 folds (w/v) saline and homogenated. Plasma and brain samples were stored at −20 °C for further analysis.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) assay
For both clinical and animal sample, 20 µL standard samples in single, quality control samples in duplicate, and samples to be tested were mixed with 60 µL acetonitrile containing internal standard (50 ng/mL of propranolol, 200 ng/mL of tolbutamide and 500 ng/mL of diclofenac sodium) in Eppendorf tubes. The mixture was then vortexed for 1 min and centrifuged for 10 min (13,000 rpm, 4 °C). 50 µL of supernatant was mixed with 150 µL purified water and shaken for 10 min. Two µL of plasma sample, 5 µL of brain or CSF sample were injected into LC-MS/MS system for analysis. The method for LC-MS/MS system was previously reported (17).
Statistical analysis
Overall survival was calculated from time of diagnosis to death of any cause or, for the patients alive, on the date of the last follow-up. In inhibition assay, the IC50 value (half maximal inhibitory concentration) was calculated using the GraphPad Prism 6 software.
Results
PCNSL patients achieved good clinical response after ibrutinib treatment
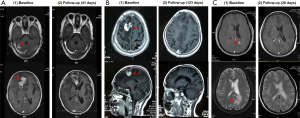
Three PCNSL patients with lesions in brain parenchyma were treated with ibrutinib monotherapy or in combination with other regimens either as first-line or sequential treatment (Table 1). Two patients (patient #1 and #3) were examined for MYD88 mutation. MYD88 L265P was found to be present in both patients. Two PCNSL patients (patient #1 and #2) were treated with ibrutinib monotherapy as first-line therapy, because they were both elderly and unfit for chemotherapy and radiation therapy. Patient #3 has recurrent PCNSL and received chemotherapy based on rituximab and methotrexate in the previous treatment. Patients #1 achieved PR while patients #2 and #3 achieved CR after ibrutinib treatment, which was indicated by the shrinkage of lesion in brain (Figure 1).
Table 1
| ID | Age, years | Gender | COO | MYD88 | Prior treatment | Disease status | Ibrutinib-based therapy | Line of therapy | Best response | Status | PFS (month) | OS (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 79 | Male | NGCB | L256P | None | Treat-naive | Ib | 1 | PR | Dead | 5 | 5 |
| #2 | 76 | Female | GCB | N/A | None | Treat-naive | Ib | 1 | CR | Dead | 5 | 7 |
| #3 | 45 | Female | NGCB | L256P | Chemotherapy | Recurrent | MTX + Ib | 2 | CR | Alive | 11 | 16 |
Patient #1 died of accidents irrelevant to the disease. Patient #2 took the medicine at 560 mg once daily for 67 days, then stopped medication due to financial reasons. COO, cell-of-origin (as determined by immunohistochemistry); NGCB, non-germinal center B cell-like; GCB, germinal center B cell-like; N/A, not assessed; MTX, methotrexate; Ib, ibrutinib (560 mg); PR, partial remission; CR, complete remission; PFS, progression-free survival; OS, overall survival.
To measure the concentration of ibrutinib in CNS, CSF of patient #3 was collected at 2 hours after 560 mg ibrutinib oral administration. Drug concentration was 0.309 ng/mL. The result indicated that at this dosage, ibrutinib is able to pass through the blood brain barrier (BBB) and achieve a concentration at which ibrutinib showed satisfactory therapeutic effect against lymphomas in brain parenchyma.
Antitumor effect of ibrutinib and other BTK inhibitors: zanubrutinib and tirabrutinib
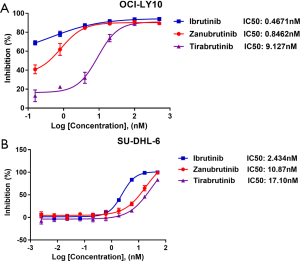
Based on the good clinical response to ibrutinib treatment, we sought to determine the anti-tumor effects of second-generation’s BTK inhibitors, zanubrutinib and tirabrutinib, by performing cell inhibition assay in DLBCL cell lines, SU-DHL-6 and OCI-LY10. IC50 was then calculated. In SU-DHL-6 cells, ibrutinib exhibited the best anti-tumoral effect among three inhibitors, with a IC50 of 2.434 µM (n=2), while zanubrutinib and tirabrutinib showed inferior effects, with IC50 of 10.87 µM (n=2) and 17.10 µM (n=2), respectively (Figure 2A). Similarly, in OCI-LY10 cells, ibrutinib had a better tumor killing efficacy, with the IC50 of 0.4671 µM (n=2), whereas zanubrutinib and tirabrutinib had the IC50 of 0.846 µM (n=2) and 9.127 µM (n=2) respectively (Figure 2B). Overall, all three BTK inhibitors were found effective against DLBCL cells. Among them, ibrutinib exerted the best anti-tumoral effect, yet tirabrutinib exerted the worst.
High dose of BTK inhibitors induce apoptosis of DLBCL cells
Apoptosis assay was then performed to investigate the effects of three BTK inhibitors on inducing cell apoptosis. As shown in Figure 3, the abilities to induce apoptosis in SU-DHL-6 cells differ among different BTK inhibitors. After 24 hours, cells treated with ibrutinib began to undergo apoptosis. After 48 hours, cells further subjected to apoptosis, showing increased number of dead and late apoptotic cells (Figure 3A). This effect was prominent in cells treated with high-dosage ibrutinib (50 µM) and subtle at a lower concentration (5 µM), indicating a time- and dose-dependent manner of ibrutinib-induced apoptosis. Apoptotic behavior was not observed when cells were treated with low concentration of ibrutinib (0.5 µM).
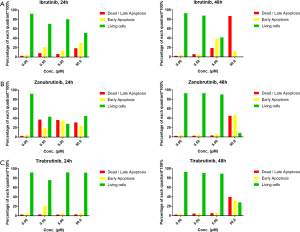
When co-incubated for 24 hours, zanubrutinib exhibited good ability in inducing cell apoptosis at the concentration of 0.5 µM or higher, in a dose-independent manner (Figure 3B). However, at lower concentration, cells recovered from apoptosis after another 24 hours, whereas zanubrutinib’s pro-apoptosis effect maintained at the concentration of 50 µM. As for tirabrutinib, however, cell apoptosis was only induced when cells were treated with high dose tirabrutinib for a relatively longer period of time (50 µM, 48 h) (Figure 3C). These results suggest that ibrutinib, zanubrutinib and tirabrutinib have different efficacy in inducing cell apoptosis, which are generally consistent with their abilities in cell growth inhibition. Higher dosage and prolonged administration contribute to better pro-apoptotic behavior of DLBCL cells.
Ibrutinib and tirabrutinib might be more suitable for brain-confined diseases than zanubrutinib
As indicated by the inhibition and apoptosis assay, high level of drug concentration in a prolonged period is required for the complete inhibition of tumor cells. Therefore, to determine the feasibility of BTK inhibitors in treating PCNSL, we tested if they were able to maintain the concentration in brain efficient for tumor inhibition. SD rats were orally administered with BTK inhibitors once. Plasma and brain tissue were sampled at designated time points to test the drug concentration (Figure 4). As indicated by Figure 4, all three BTK inhibitors were rapidly absorbed into plasma and brain. Both unbound ibrutinib and tirabrutinib reached Cmax 2 hours post administration, and maintained at a relatively stable level, both in blood and brain tissue (ibrutinib: blood Cmax =412.7 ng/mL, brain Cmax =40.4 ng/mL, n=3; tirabrutinib: blood Cmax =339.53 ng/mL, brain Cmax =28.9 ng/mL, n=3). As for unbound zanubrutinib, it rapidly reached maximum concentration in blood and brain at 0.5-hour post administration, and exhibited a decrease in blood concentration slightly faster than the other BTK inhibitors. In brain, on the other hand, unbound zanubrutinib concentration slumped, and was below the limit of detection 4 hours after administration.

Because Cmax in blood and brain were simultaneously reached by each BTK inhibitor (ibrutinib and tirabrutinib: 2 hour post oral administration; zanubrutinib: 0.5 hour post oral administration), unbound brain-to-plasma concentration ratio was calculated at the time they reached Cmax. Unbound brain-to-plasma concentration ratio of zanubrutinib, tirabrutinib and ibrutinib were 3.5%, 8.5% and 9.8%, respectively (Table 2). This ratio of ibrutinib is slightly higher than tirabrutinib. This ratio of zanubrutinib, however, was much lower than ibrutinib and tirabrutinib, indicating its inferior ability to pass through BBB compared to ibrutinib and tirabrutinib.
Table 2
| Cmax (ng/mL) | Zanubrutinib (15 mg/kg orally) | Tirabrutinib (10 mg/kg orally) | Ibrutinib (55 mg/kg orally) |
|---|---|---|---|
| Plasma, Cmax (ng/mL) | 884 | 339.53 | 412.7 |
| Brain, Cmax (ng/mL) | 30.9 | 28.9 | 40.4 |
| Penetration rate (%, Cmax, brain/Cmax, plasma) | 3.5 | 8.5 | 9.8 |
Together, these data indicate that ibrutinib, tirabrutinib and zanubrutinib can be rapidly absorbed into blood and distributed into brain after oral administration. Compared with zanubrutinib, ibrutinib and tirabrutinib exerted better ability in passing through BBB and maintaining a high and stable concentration in brain, facilitating these inhibitors to exert their anti-tumoral effect in brain, making them more promising candidates for the treatment of PCNSL.
Discussion
BTK is a tyrosine kinase downstream of the pre-B-cell receptor and B-cell receptor, especially essential for the survival of lymphoma with chronic active NF-kB pathway. BTK inhibitors disrupt BCR downstream signaling and induce apoptosis of PCNSL (18). In this work, retrospective study identified 3 patients who received ibrutinib-based therapy, who are either with refractory and recurrent PCNSL or unfit for chemoradiotherapy. Although two of them died unfortunately, lesions identified by MRI image shrunk after ibrutinib treatment, suggesting good clinical response. In clinical trial with larger cohort, clinical responses to ibrutinib occurred in 10 of 13 (77%) in patients with refractory and recurrent PCNSL and five patients achieved CR (11). Also, in patients with refractory and recurrent PCNSL, a phase II study demonstrated 70% disease control rate in evaluable patients and 62% in the intent-to-treat analysis including 19% CR, 33% PR and 10% stable diseases achieved by patients receiving ibrutinib monotherapy (560 mg/day). Ibrutinib concentration in CSF were measured in this study and the mean concentration was 0.23 ng/mL (0.2–0.84 ng/mL) on day 29 (10), consistent with the drug CSF concentration acquired in this study (0.309 ng/mL). Taken together, our study further supports the use of ibrutinib in PCNSL patients based on its good effect and sufficient BBB penetration. In view of this, ibrutinib-based regimen could be a good candidate for patients with refractory and recurrent PCNSL or treat-naïve patients with poor physical condition or unfit for chemoradiotherapy.
As ibrutinib yields relatively high toxicities due the off-target effects, new generation of BTK inhibitors with long-term effectiveness and less toxicity have been developed, which might make them better candidates for PCNSL. Zanubrutinib has entered several clinical trials, showing extraordinary therapeutical efficacy in mantle cell lymphoma, thus being approved by US Food and Drug Administration for its treatment (13-15,19). Similarly, tirabrutinib was also examined in clinical trials for patients with B-cell malignancies (20,21). To further evaluate their role in PCNSL, we compared their tumor suppressing abilities in DLBCL cells since the majority of PCNSL are DLBCL (2). As indicated by the result, ibrutinib and zanubrutinib have comparable ability in cell inhibition, better than that of tirabrutinib. Also, apoptosis assay suggested that the induction and sustainability of apoptosis required prolonged treatment of BTK inhibitors at a relative high dosage. Therefore, it is vital to determine their abilities in sustaining a desirable amount of drug in brain so as to fully exert their tumor killing effect. In vivo study suggested that, speaking of brain-confined lymphoma, the pharmacokinetic properties of tirabrutinib and ibrutinib might made them better drug candidates, because they both could rapidly penetrate through BBB and distribute in brain tissue, while zanubrutinib failed to sustain high level of drug in the brain parenchyma. Since steady concentration of BTK inhibitors in the brain parenchyma is a necessity for the elimination of brain-located tumors, pharmacokinetic parameters in the brain prefer to support the use of tirabrutinib and ibrutinib in PCNSL, even though zanubrutinib exerted better tumor killing ability in vitro. At the meantime, the result of a phase I/II trail in relapsed/refractory PCNSL patients was open to public, suggesting that tirabrutinib does show favorable efficacy in PCNSL patients, contributing to a good ORR rate of 64% (22). Consistent with our findings, this clinical trial further supports the use of BTK inhibitors in PCNSL.
However, it should be noted that choice of these inhibitors clinically cannot fully rely on results of pre-clinical studies. Owing to the difficulties in preparing animal models of PCNSL, we were unable to test the tumor suppressing ability of BTK inhibitors in vivo, which has made our findings rather indirect proof for inhibitor selection. Also, although the results suggested that among three inhibitors, ibrutinib ranked first in candidate against PCNSL, it is possible that tirabrutinib may actually be better in treating PCNSL since the evaluation of systemic toxicities and adverse effects was not included in this study. In fact, tirabrutinib exhibited better kinase selectivity for BTK against other enzymes like BMX, EGFR, and ITK compared to ibrutinib (12). And as we know, off-target inhibitions by BTK inhibitors are believed to contribute to its adverse events. Also, previous researchers have discovered that zanubrutinib has improved pharmacologic properties compared to ibrutinib, including oral bioavailability and selectivity for BTK (15,16,23). Therefore, comprehensive and clinical investigations are needed for the all-round evaluations of BTK inhibitors.
Conclusions
In conclusion, our study verified that PCNSL patients could benefit from BTK inhibitor ibrutinib. In vitro study indicated that zanubrutinib, tirabrutinib and ibrutinib were all effective in inhibiting the proliferation and inducing the apoptosis of DLBCL cells, especially ibrutinib. In SD rats, three inhibitors successfully penetrated into brain, while tirabrutinib and ibrutinib exerted better pharmacokinetic properties in brain, suggesting a preference for these inhibitors in PCNSL treatment. In all, these findings support the clinical applications of BTK inhibitors in PCNSL, and provide important information for the selection of optimal treatment regimens. These findings encourage further exploration of novel BTK inhibitors and drug combinations, either in frontline or multiline treatment for the benefit of PCNSL patients.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-21-50
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-21-50
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-21-50) and reported that this work was supported by Science and Technology Program of Traditional Chinese Medicine, and Zhejiang(2021ZB038) and Zhejiang Provincial Medicine and Health Science Fund(2021KY105). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Study involving human subjects was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital. (IRB-2019-3). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients gave written informed consent to participate in the study. Studies involving animals were approved by Ethics Committee for Experimental Animals of Zhejiang Cancer Hospital (2020-12-004). All animals were handled in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011;105:1414-8. [Crossref] [PubMed]
- Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol 2017;35:2410-8. [Crossref] [PubMed]
- Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol 2006;80:159-65. [Crossref] [PubMed]
- Lange M, Rigal O, Clarisse B, et al. Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer Treatment Reviews 2014;40:810-7. [Crossref] [PubMed]
- Weiss B. Chemobrain: a translational challenge for neurotoxicology. Neurotoxicology 2008;29:891-8. [Crossref] [PubMed]
- Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol 2015;52:67-76. [Crossref] [PubMed]
- Mohamed AJ, Yu L, Bäckesjö CM, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 2009;228:58-73. [Crossref] [PubMed]
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6. [Crossref] [PubMed]
- Goldwirt L, Beccaria K, Ple A, et al. Ibrutinib brain distribution: a preclinical study. Cancer Chemother Pharmacol 2018;81:783-9. [Crossref] [PubMed]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 2019;117:121-30. [Crossref] [PubMed]
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018-29. [Crossref] [PubMed]
- Liclican A, Serafini L, Xing W, et al. Biochemical characterization of tirabrutinib and other irreversible inhibitors of Bruton’s tyrosine kinase reveals differences in on - and off - target inhibition. Biochim Biophys Acta Gen Subj 2020;1864:129531. [Crossref] [PubMed]
- Song Y, Zhou K, Zou D, et al. Treatment of Patients with Relapsed or Refractory Mantle–Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton’s Tyrosine Kinase. Clin Cancer Res 2020;26:4216-24. [Crossref] [PubMed]
- Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol 2020;13:48. [Crossref] [PubMed]
- Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019;134:851-9. [Crossref] [PubMed]
- Tam CS, Opat S, Cull G, et al. Twice Daily Dosing with the Highly Specific BTK Inhibitor, Bgb-3111, Achieves Complete and Continuous BTK Occupancy in Lymph Nodes, and Is Associated with Durable Responses in Patients (pts) with Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL). Blood 2016;128:642. [Crossref]
- Yang H, Li C, Chen Z, et al. Determination of chidamide in rat plasma and cerebrospinal fluid. Regul Toxicol Pharmacol 2018;98:24-30. [Crossref] [PubMed]
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88-92. [Crossref] [PubMed]
- Syed YY. Zanubrutinib: First Approval. Drugs 2020;80:91-7. [Crossref] [PubMed]
- Munakata W, Ando K, Hatake K, et al. Phase I study of tirabrutinib (ONO-4059/GS-4059) in patients with relapsed or refractory B-cell malignancies in Japan. Cancer Sci 2019;110:1686-94. [Crossref] [PubMed]
- Rule SA, Cartron G, Fegan C, et al. Long-term follow-up of patients with mantle cell lymphoma (MCL) treated with the selective Bruton’s tyrosine kinase inhibitor tirabrutinib (GS/ONO-4059). Leukemia 2020;34:1458-61. [Crossref] [PubMed]
- Narita Y, Nagane M, Mishima K, et al. Phase 1/2 Study of Tirabrutinib, a Second-Generation Bruton’s Tyrosine Kinase Inhibitor, in Relapsed/Refractory Primary Central Nervous System Lymphoma. Neuro Oncol 2021;23:122-33. [Crossref] [PubMed]
- Tam C, Grigg AP, Opat S, et al. The BTK Inhibitor, Bgb-3111, Is Safe, Tolerable, and Highly Active in Patients with Relapsed/ Refractory B-Cell Malignancies: Initial Report of a Phase 1 First-in-Human Trial. Blood 2015;126:832. [Crossref]


