Historical perspective and evolution of charged particle beam therapy
History of charged particle beam therapy
Since Roentgen’s discovery of X-ray 130 years ago, radiation has been used to treat various diseases including cancer (Figures 1,2). As much excitement seen in the early days when X-ray was found to be effective in shrinking tumors, it was also realized of the potential severe early and late adverse complications from the same treatment to nearby normal structures.

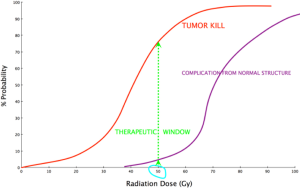
The early observations lead to further research and establishing one of the fundamental principle of radiotherapy, called “the therapeutic window”, which compares the degree of tumor kill to the damages of surrounding normal structures (Figure 3).
The proton was discovered by Ernest Rutherford in the early 1900’s. By irradiating nitrogen gas with (alpha) particles would lead to oxygen atoms and the dense nuclei of hydrogen atoms, which he named protons based on the Greek word “protos” which meant first. His conclusion was that the nitrogen atom was made up of some number of protons and electrons and can be transmuted into oxygen and a hydrogen nucleus. It was also discovered that charged particles (protons and light ions) have a finite range in matter. The interaction probability to cause ionization increases as they loose velocity along their paths, so that a peak of deposited dose occurs at a depth proportional to the energy of the charged particle. Beyond this peak, no further dose is deposited. This scientific phenomenon was described the William Bragg at that time (1). In 1930, the American physicist Ernest O. Lawrence and his associates were the first to invent cyclotron to accelerate proton to the energy high enough for cancer treatment applications. He invented the cyclotron in 1929 & developed it as a particle accelerator during the 1930s, winning the 1939 Nobel Prize for physics for this work. In 1931 he founded the Radiation Laboratory, later the Lawrence Berkeley Laboratory (Figure 4). In a decade later, his advanced version of the synchrocyclotron, which has 184-inch in diameter, is capable of producing 340 MeV protons (Figure 5) (2).

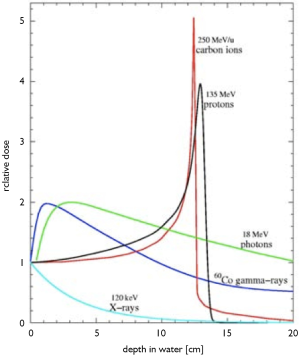
In 1946, Dr. Robert Rathburn Wilson wrote a seminal paper proposing the idea that proton beams could be used for cancer treatment (3) while he was in the Physics Department at Harvard University. He described the fundamental physical feature depth-dose curve of proton and heavy-charged particles in comparison with photon or X-rays. He described the way the particle beams deposit their energy as the beam enters the body en route to the tumor: smaller amount of energy is released first, and then much larger amount of the beam energy released at the end of its path (Bragg peak) and completely stops (Figure 6). The depth at which the particle beam stops can be controlled within millimeter precision by adjusting the beam energy using a rotating wheel of variable thickness, i.e., a range modulation wheel (RMW). This technique is still being used today, and it is a simple way of adding multiple Bragg peaks of variable energies and weights in order to spread the proton stopping region over the tumor volume. He did also play significant role in the development of nuclear weapons during World War II (“The Manhattan Project”); but afterwards, he chose to shift his focus of nuclear physics into medical application for the betterment of mankind. In addition to Wilson’s being a very accomplished sculptor and architect, he was later responsible for the development of Fermi Laboratory and became its founding director (Figure 7).

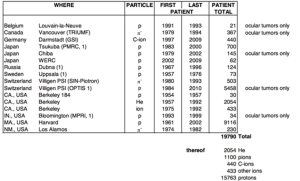
Prior to treating human, the first investigations on biological effects of protons were done on rodents by Cornelius Tobias and John Lawrence (brother of Ernest O Lawrence) using this 184-inch synchrocyclotron during the late 1940’s and early 1950’s (4). The first patients treated by protons was at the University of California, Berkeley in 1954 by the Lawrence “boys” (as they preferred to be called). The initial tumor targeted at was pituitary tumor since it could be located in 3-D using orthogonal plane X-ray films and rigid immobilization of the cranium (5). This was done some 20 years before the invention of CT scan. This isocentric technique forms the basis for stereotactic radiosurgery for many decades later. From 1954 to 1957, a total of thirty patients was treated with proton beam here. Due to better understanding of higher linear energy transfer (LET) and radiobiological equivalent (RBE) of heavier charged particle. In 1957, this synchrocyclotron was modified to accelerate Helium nuclei. By the time of the facility’s closure in 1992, a total of 2,054 patients had been treated with helium ions. Concurrently, another particle beam facility was being developed in Uppsala, Sweden where they treated their first proton patient in 1957. The MGH-Harvard Cyclotron Laboratory was open in 1961, and then the Institute for Theoretical and Experimental Physics in Moscow in 1967. Over the next few decades, about 10 more facilities were open around the world. All these facilities were in physics laboratories with minimal infrastructure for patient care and support. Most of the patients treated at these facilities had intra-cranial or ocular tumors (Figure 8).
During the 1970’s and 1980’s, the primary technological development was the construction of the Bevatron at LBNL, a synchrotron-based facility that could accelerate charged particles ranging from helium ions to uranium nuclei. Otherwise, most of the scientific advancement in the delivery and technology of particle beam therapy in this era resulted from continuing to accumulate clinical data on selected tumor sites. These included ocular tumors, brain and base-of-skull tumors, and then pediatric malignancies. During this period, due to the emergence of CT and MRI technologies and treatment planning algorithms and softwares, better 3-D targeting and treatment planning refined the delivery and verification of proton therapy. In parallel to the development of particle beam technology, there was major advancement in photon therapy, including the emergence of intensity-modulated radiation therapy (IMRT) in the late 1990’s.
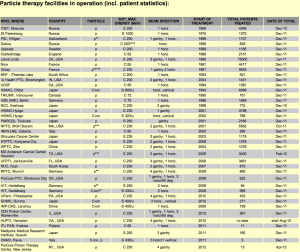
In 1990, the world first hospital-based proton therapy facility was built in Loma Linda. This effort was led by Dr. James Slater with the support from Loma Linda University Medical Center (LLUMC) and a government grant. The facility houses a Fermi Lab-designed 250 MeV Synchrotron, a passive-beam nozzle, and four treatment vaults with three rotating gantries and one fixed beam room. This patient-dedicated center has treated the most patients with particle beam to date, more than 15,000 patients. Up to the late 1990, most patients treated with particle beam in the U.S. were considered “experimental” or “investigational” by Medicare and private insurance carriers; one of significant and non-scientific event that has popularized the use of proton therapy in the U.S. is the approval of proton-specific treatment delivery procedure codes from American Medical Association (AMA). This effort was led by LLUMC and Massachusetts General Hospital (MGH). When this reimbursement rate was set by Medicare, the wording of “investigational” and “experimental” were removed from the domain of proton beam therapy. Like almost all technologies in radiation therapy, this approved reimbursement rate has provided financial incentives for a wave of hospitals and private sector in the U.S. to consider proton therapy due to the attractive rate of return of the investment. Concurrently, significant publications started to emerge on the superior clinical outcome of proton on clinical sites such as pediatric, eye, base of skull and spinal tumors. In the same period, there were availability of vendors offering commercial solutions for development of these facilities. These factors have led to the rapid implementation of clinical centers in the U.S., Asia and Europe. This Figure (reference to PTCOG website as of Sept 24, 2012) shows the current particle beam facilities in the world. According to the PTCOG, the number of patients being treated with particle beam has recently exceeded 100,000 at 38 charged particle beam therapy centers worldwide (Figure 9).
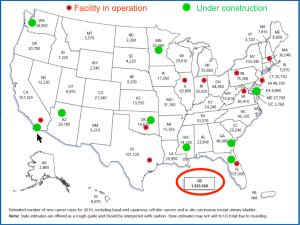
In the U.S., there are currently ten centers in operations, and almost another ten facilities are under construction or being planned as shown geographically in Figure 10. With an approximate 1.5 million new cases of cancer in the U.S each year, and approximate half of those patients (about 750,000) will receive radiation therapy as part of their treatment. According to an analysis by Dr. James Cox, roughly about 15% will be the candidate for particle beam therapy, or about 100,000 cases annually in the U.S (6). More than 95% of these cancer patients currently receive their radiotherapy at over two thousands photon radiation facilities in the U.S.
Evolution in charged particle beam therapy
Over the past 100+ years, the field of radiation therapy has progressed over the three frontiers: technology, radiobiology and clinical trials. These principles also apply to charged particle therapy. In the following sections, the evolution, challenges, and future direction will be discussed.
Technological and scientific evolution of charged particle beam therapy
Comparing to other fields in medicine, radiation therapy (including charged particle beam therapy) has depended most heavily on technology and science for its advancement. There have been continuing development in many aspects of technologies to discover various sources and mode of radiation and transitioned from the physics laboratory into the clinical setting. The cooperation among research laboratories, academic medical centers, and private industries have continued to improve the technology, affordability, and ease of implementation. There are three areas that will be discussed in the details in the following sections.
Generation and delivery of charged particle beam
The fundamental principle of radiotherapy is the use of ionizing radiation to selectively damage and/or kill diseased cells at certain geometric target for some end clinical result with reasonable collateral damage of surrounding non-disease structures. The advancement of ionizing radiation is all about the discovery of reliable new types or sources of ionizing radiation with desirable physical characteristics that can be delivered to a human being for this principle. The field of radiation has evolved from low-energy X-rays to natural Cobalt-60 gamma rays, then bremstralung rays from electron linear accelerator in the MeV range, followed by modern day linear accelerator that generates photon in the 10 to 20 MV range. As discussed in the Introduction Section, the early scientists had discovered types of charged particles, how to accelerate their energy to achieve a certain useful clinical depth, how to deliver these particles precisely into a three-dimensional target volume inside human, and how to plan and verify this treatment process. The components of the clinical particle beam center consists of:
(I) Accelerator
The accelerator (the “engine”) accelerates charged particle beam from rest to a range of hundreds of MeV. Traditionally, two main types of accelerator are cyclotron and synchrotron, which are different physically on how they accelerate charged particles. There are historical differences and reason why each design was chosen. They both have advantages and disadvantages, and the discussion is beyond the scope of this article, and readers are referred to article by Dr. Al Smith (7). Newer generation of accelerators are superconducting cyclotrons and synchrocyclotrons, which have higher energy, more compact size, less power consumption, and higher beam extraction efficiency.
(II) Beam transport and energy selection system
The high energy charged particles will leave the accelerator at about 2/3 the speed of light and travel in vacuum in a very tightly collimated beamlet (few millimeters) to the treatment room. This part of the system will assure the beam quality, direct the beam to the right treatment room, and able to change the beam energy (to allow for different tissue penetration) at the timeframe of mili-second.
(III) Beam nozzle design
This very critical component will control the deposition of the charged particle in the patient in four dimensions: spatial (x,y,z) and temporal (time). The nozzle design defines the beam delivery technique. There are two general categories: passive scattering and spot scanning techniques. A detailed description can be found in the literature (8). The traditional passive scattering technique deposits an uniform dose to a 3-D target using charged particles with the same energy at the same time interval. A small focused uniform beamlet is “scattered” by some material to increase the beam size. The lateral dimension (x and y) of the target was shaped using a custom cut-out or block; the depth dimension (z) of the target was shaped using a compensator, which is a “negative” or mold shape of the target; all the dose is delivered to the target in some time interval of minutes. This is almost similar to the 3-D conformal treatment with photon. The passive scattering technique accounts for most of the current clinical data in particle beam therapy. It has several advantages: less expensive and easy to implement on the hardware; more “forgiving” for organ motion; easy treatment planning and plan verification. There are many disadvantages of the passive scattering technique: (i) Passive scattering technique does not use the charged particles efficiently. The majority of the particles generated is wasted due to scattering and never reach the target; (ii) There is high contamination of neutron generated due to these scattering particles. These neutrons are putting patients (particularly young patients) at risk for secondary malignancies later in life; (iii) The passive scattering technique requires fabrication of the 3-D compensators and block for each field in each patient. These add time and cost to the treatment process. These devices have to be re-made each time there is a change in target size and shape; (iv) This technique treats the normal structure proximal to the target unintentionally; (v) Due to multiple scattering of the beam, the lateral penumbra of the field is increased (larger effective source size).
To unleash more potentials of charged particle therapy, these deficiencies should be addressed, and hence the rationales for the spot scanning technique or pencil beam scanning technique. In this technique, the Bragg peak of each beamlet from the accelerator is delivered into each voxel of the 3-D target. There are no scattering devices, and the 3-D placement (x,y,z) of these beamlets are done using scanning magnets and energy changes on the fly. A target with one liter in volume could contain around 10,000 or more voxels. The dose deposit to each voxel last few milliseconds and the whole process take about 1-2 minute for a 2 Gy dose. There are many versions (and names) of the spot scanning techniques with varying sophistication of the system. The most advanced spot scanning technique is intensity-modulated particle beam therapy (IMPT), where multiple pencil beam scanning fields are optimized simultaneously to produce a desired dose distribution to a 3-D target. IMPT allows for variation in beam energy and intensity. Overcoming the disadvantages of passive scattering technique, the newer spot scanning technique does present with challenges: (I) Treatment planning and verification are much more complicated; (II) Sensitive to organ motions; (III) More expensive and difficult technology to implement, particularly the control system; (IV) More clinical data are needed. More research and works are currently being done to address these challenges. There are also availability of “universal” “nozzle” which has ability to deliver both passive scattering and spot scanning techniques.
(IV) Patient positioning and verification system (PPVS)
The significant difference between a clinical and research facility is the patient positioning and verification system, which allows for accurate and comfortable patient setup and verification of dose delivery process. Robotics and automation have been introduced into the clinical facilities in the past decade to allow for improved patient transport, facility throughput, better patient positioning, and accurate setup.
(V) The control system
The control system (“the brain”) is the most critical component of the clinical treatment facility. The control system involves in all steps of the treatment process to achieve a safe and accurate treatment to patients and protect the personnel. This system interacts with accelerator, treatment planning software, electronic medical record (EMR), the treatment control and dose monitoring at the treatment nozzle, patient positioning and verification system, electronic/magnetic components in the facility, radiation detection monitor system, and mechanical component (door sensor, collision system, etc.). The control system is the ultimate safety defense for a clinical facility and must be 100% functional.
Treatment planning software (TPS)
The advancement of charged particle therapy will not be at this stage if there are not such parallel progress in treatment algorithms, softwares, and computers. Unlike other local treatment modalities for cancer, TPS allows the radiation oncologists optimize for the best plan and double check it before the treatment is delivered. TPS models the actual dose deposition in the patients and automation by going over thousands of possible combinations of treatment parameters to derive the best plan. Comparing to photon treatment planning, charged particle beam therapy planning is more complicated due to the followings: (i) Tissue inhomogeneity and interfaces (bone/air, tissue/metallic prosthesis) can affect dose distribution significantly; (ii) Due to the finite range of charged particles, the tissue-equivalent distance obtained from imaging studies such as CT and/or MRI should be looked at carefully; (iii) Treatment planning for spot scanning beam with organ motion is challenging. As the newer technologies emerge for charged particle beam therapy, the demand for better and faster TPS and computers goes up. The current developments for TPS include: (i) more advanced calculation algorithms such as Monte Carlo modeling is preferred over traditional ray-tracing algorithm; (ii) accounting for organ motion; (iii) adaptive therapy to accommodate for the change in tumor size and surroundings during the course of treatment; (iv) image-guided radiation therapy; (v) plan robustness modeling to account for treatment uncertainty parameters; (vi) biological modeling to account for biological dose and effects; (vii) more objective plan evaluation to automating the treatment planning process; (viii) in-vivo dose verification using the information from gamma camera and modeling of PET emitters; (ix) modeling of small field dosimetry for stereotactic radiosurgery and stereotactic body radiation therapy applications.
Radiological imaging
Parallel with advances in technology and treatment planning software, imaging technologies and faster computers, progress in the field of radiological imaging such as CT, MRI, PET has provided advancement to the field of charged particle therapy. Better 3-D anatomical and functional imaging technologies help clinician better defining the target and critical structures, adapting the treatment plan to tumor response during the treatment course, and providing feedback via evaluating the tumor response and complications after treatment completion. As 2-D and 3-D anatomical imaging modalities such as kV radiographs, CT, and MRI are incorporated into the treatment planning and verification. The resolution of these imaging systems will guide the accuracy of the dose delivery system. Current research work is focusing on the use of functional imaging such as PET for charged particle therapy as a way of dose recording and verification system. This is based on the fact that high-energy charged particles interact with human tissue and produce positron emitters or PET isotopes (Carbon-11 and Oxygen-15 with a half-life of 20 and 2 minutes, respectively). The PET activity can be characteristically related in 3-D to the dose delivered and biological effects. In-room PET camera imaging system has been developed to investigate this property (9).
Radiobiology of charged particle beam therapy
The amount of radiation deposition into tissue is measured by a physic quantity, called Gray (or Gy). One Gray is defined as the amount of energy (measured in Joule) deposited in a unit of tissue mass of one Kilogram. This measurable unit of dose in radiation therapy does not tell or predict what happens at the molecular or cellular level. Radiobiology is the field of science that connects the dot between deposited dose to clinical endpoint. This basic science provides us the understanding the biological effects of radiation at cellular levels, repair mechanism, and multiple interactions with oxygen level, micro- and macro-environment, and differential effects on various tumor types and normal tissues. If one follows the track of ionizing radiation as it enters a human body, the radiation ray gives up its energy and causing ionization to tissues, which are then translated into the radio-biological effects (RBE). The amount of radiation transferred per unit track is described by a quantity called Linear Energy Transfer (LET). For a given type of radiation, RBE can be thought of as a conversion factor from the dose deposited in Gray into the some biological effects. The physical dose in Gray is what we can measure with instrumentation, but the end biological effects are of significant interests (“biological effectiveness”). For each radiation type, RBE depends several factors such as type of radiation (hence LET), the speed of the particle, tissue types, and the local micro-environments (oxygen level, etc.). Low LET radiation (or sparsely-ionizing radiation) such as photon or X-ray transfers much less ionization along the path; therefore, their RBE is low, and defined as 1.0. High LET radiation (or densely-ionizing radiation) such as carbon, helium, neon particles transfers more energy along its path, and significantly more at the end of the Bragg peak; therefore, their RBE is higher (range, 1.5-4). For example, for a sample amount of 1 Gray dose to a target, carbon beam with RBE =3 will have three times the biological effects as photon beam with RBE =1. Higher RBE radiation is more effective against radio-resistant and hypoxic tumors since they are more likely to cause cell injuries by double-strand breaks and clustered damages in the DNA. Proton beam has RBE slightly higher than photon, about 1.1 to 1.2, and is considered to be a low LET type of radiation. It is also worth noting that the value of RBE varies along the path of particle beam, higher at the Bragg peak are than plateau area, and this difference is higher for heavier charged particle. From the theoretical standpoints, heavier charged particles such as Carbon and Helium have advantages over proton due to: (i) their Bragg peak is more pronounced; (ii) they have higher RBE, hence more effective against hypoxic or radio-resistant tumors and better cell-cycle independent cell kill; (iii) they have sharper beam edge or lateral penumbra. Disadvantages of using heavier charged particle therapy are: (i) the equipment is more expensive; (ii) the fragmentation region (or the stopping distance beyond the Bragg peak) is greater than proton; (iii) clinical experience is less than proton; (iv) RBE varies over the path of the beam; and incorporating this bio-effectiveness into treatment planning is difficult.
Clinical trials in charged particle beam therapy
Since its discovery, radiation had been used for many other diseases beside cancer such as acnes, infection, hair loss, arthritis, etc. Unfortunately, lots of lessons of the inappropriate use of radiation had long-lasting and severe complications that had brought a negative image to the field. In the early day, the use of radiation (and other medical treatments) was up to the call of the practitioner, and lot of knowledge was not transferrable or reproducible. Formation of clinical studies have significantly advanced the medical field (and protecting the patients). Multi-center clinical studies through such organization as Radiation Therapy Co-Operative Group (RTOG) have defined the standard of care in radiation therapy. The well-conducted clinical trials have defined the appropriate disease/stage, the technique (dose, fractionation, constraints), interactions with other modalities (surgery, chemotherapy), and outcomes. As the number of clinical centers for charged particle beam increases and emergence of more sophisticated technologies, the clinical application of charged particle beam therapy is expanded to more tumor sites from traditional applications such as tumors at the orbits, base of skull, spine, and pediatric population. A recent effort of PTCOG to compile clinical trials in charged particle beam therapy was reported by Giap et al. (10,11) and listed at the PTCOG website. There are more than 60 clinical studies investigating tumors of various sites from ten centers. The compiled list of clinical protocols shows a diversified potential application in cancers of the lung, head and neck, gastrointestinal tract, prostate, breast, brain, gynecologic sites, lymphoma, and recurrent tumors. These clinical trials will validate or invalidate the use of particle beam for these disease sites. The publication of clinical trials will enhance awareness and accelerate the patient accrual to provide the answers. The other benefit of these listings will be for clinicians who are planning new clinical trials basing on these information and to promote the collaboration among multi-centers in conducting these clinical studies. In the U.S., due to its higher cost, charged particle beam therapy has faced lots of pressure from government, insurance carriers, and photon treatment centers to justify its superiority over the conventional radiation treatment. With the Agency for Healthcare Research and Quality (AHRQ) from the government promotes Comparative Effectiveness Research (CER) for various treatment modalities for a given type of disease. CER is designed to inform healthcare decisions by provide evidence on clinical effectiveness, benefits, and side effects of different treatment options. All modalities of cancer treatment will have to produce these data to justify itself. Clinical studies will provide these data and identify a subset of cancer patients that would best be served by charged particle beam therapy either by more effective in local control and/or less side effects and/or both.
Conclusions
This article provides a historical perspective of charged particle therapy over the last 80 years. As cancer becomes the leading cause of mortality and morbidity in the U.S. and the rest of the world, and majority of cancer death and suffering is due to insufficient local control of tumor. Radiation therapy including charged particle therapy has been and will continue to be an effective modality for cancer therapy. There is still room for improvement in cancer care since only roughly half of cancer patients are cured from their diseases. There are many emergent treatment modalities for cancer therapy, and there are many refinements of existing treatments. Charged particle beam therapy has come a long way based upon its fundamental physical advantages, although more sophisticated, computer-intensive improvements are actively being pursued. Any strategy that continues to rely exclusively on using spread-out Bragg peak techniques with passive scattering, without modernizing the delivery and planning techniques, will cause the field of charged particle beam therapy will fall behind. With the recent emergence of much-improved technology and the engagement of many major academic centers into the field, this is the time that the field of charged particle therapy should take a quantum leap forward to unleash all these potentials. Charged particle therapy will never completely replace other modalities of radiation therapy or local treatment modalities, but it has to continue to evolve and push the bar higher by producing the clinical evidence for treatment of various cancer types. Perhaps, we could learn from one of humanity’s greatest artists, Michelangelo: “The greater danger for most of us is not that our aim is too high and we miss it, but that it is too low and we reach it”.
Acknowledgments
The authors would like to express gratitude to Dr. Richard P Levy for the review and comments on this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Particle Beam Therapy I”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.10.09).The series “Particle Beam Therapy I” was commissioned by the editorial office without any funding or sponsorship. HG served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Translational Cancer Research. The author have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown A, Suit H. The centenary of the discovery of the Bragg peak. Radiother Oncol 2004;73:265-8. [PubMed]
- Lawrence E, Livingston M. The production of high speed protons without the use of high voltages. Phys Rev 1931;38:834.
- Wilson RR. Radiological use of fast protons. Radiology 1946;47:487-91. [PubMed]
- Tobias CA, Anger HO, Lawrence JH. Radiological use of high energy deuterons and alpha particles. Am J Roentgenol Radium Ther Nucl Med 1952;67:1-27. [PubMed]
- Levy RP, Blakely EA, Chu WT, et al. The current status and future directions of heavy charged particle therapy in medicine. AIP Conf Proc 2008;1099:410-25.
- Cox J. Presentation at RadOnc 2010 at University of Texas M.D. Anderson Cancer Center. March 2010.
- Smith AR. Vision 20/20: proton therapy. Med Phys 2009;36:556-68. [PubMed]
- Gottschalk B, Pedroni E. Treatment delivery systems. In: DeLaney TF, Kooy HM. eds. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams and Wilkins, 2008:33-49.
- Nishio T, Miyatake A, Ogino T, et al. The development and clinical use of a beam ON-LINE PET system mounted on a rotating gantry port in proton therapy. Int J Radiat Oncol Biol Phys 2010;76:277-86. [PubMed]
- Giap et al. Compilation of clinical trials in particle beam therapy. Presented at PTCOG 51 meeting in Seoul, South Korea, in May 2012.
- Giap F, Levy R, Giap HB. Summary of on-going clinical protocols for proton and heavier-ion therapy. Proceedings the 22nd International Conference on the Application of Accelerators in Research and Industry (CAARI) 2012. In press by American Institute of Physics Conference Proceedings Series.