
Tumor-endogenous PD-1 promotes cell proliferation and predicts poor survival in non-small cell lung cancer
Introduction
Lung cancers constitute the most common type of malignancy with the highest cancer-related mortality worldwide, among which non-small cell lung cancers (NSCLCs) take 84% (1). Surgical resection for early-stage lung cancer can improve the survival of patients (2). Unfortunately, most patients are diagnosed at an advanced stage without the opportunity for surgical treatment and have to receive comprehensive therapy, including chemotherapy and radiotherapy (3). Severe side effects of chemotherapy and radiotherapy extremely affect the life quality and survival time of patients. Immunotherapy has emerged as a promising treatment modality in most malignant tumors including advanced lung cancer (4). Immunotherapy generally can be divided into active and passive strategies. Active immunotherapy is to stimulate human immune system with immune checkpoint inhibitors (ICIs), cancer vaccines or cytokines and induce de novo cancer-specific immunity. Passive immunotherapy is to induce human immune response through directly transferring T cells. Although these strategies are based on different mechanisms, they both aim to activate tumor-specific cytotoxic T lymphocytes (CTLs) to eliminate tumor cells (5).
It is well-accepted that the immune system could inhibit or conversely promote tumor progression at different phases of tumorigenesis (6). Under normal circumstances, immune system identifies and eliminates tumor cells, the process called immune surveillance. However, mutagenesis induced by the stress of immune surveillance can provide increased cell-to-cell variations in tumor cells. The variations appear to create a condition for selected tumor cells escaping from the immune surveillance and finally lead to immune escape. Up-regulating immune checkpoint proteins is the common way (6). Therefore, ICIs targeting the crucial checkpoint protein programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have shown improved clinical outcomes in NSCLCs. In 2015, Food and Drug Administration (FDA) approved the first immune checkpoint inhibitor nivolumab for advanced NSCLC that has progressed during or after treatment with platinum-based chemotherapy (7). Recently, FDA approved immune checkpoint inhibitors pembrolizumab and Atezolizumab for the first-line treatment of selected patients with stage III NSCLC based on the KEYNOTE042 study (8).
PD-1, a type I transmembrane protein, was discovered in the screening of apoptosis-related genes, later proved to be a co-inhibitory immune checkpoint, negatively regulating immune response (9). Previous studies have demonstrated that PD-1 was mainly expressed on the activated T cells and B cells undergoing β-selection (10,11). In the process of tumor progression, some tumor cells up-regulate the expression of PD-L1. PD-L1 binding to PD-1 inhibits the activity of CTLs and the proliferation of regulatory T-cells (Treg). Therefore, PD-1/PD-L1 axis becomes the therapeutic target of ICIs (12-14).
Recent studies showed that the expression of PD-1 could be observed in tumor cells. Schatton et al., reported that PD-1 was endogenously expressed in melanoma cells, and it could activate the downstream mTOR signal pathway to promote tumor progression through the PD-1/PD-L1 axis (15). Ye et al., reported that liver cancer cells also express PD-1 endogenously, suggesting that the combination of PD-1 antibody and mTOR inhibitor can produce a more potent anti-tumor effect (16). These studies deepen the understanding of tumor immune relevance and provide new ICIs-related immunotherapy ideas. To our knowledge, the work about tumor cell-intrinsic PD-1 in NSCLCs has not been reported at present. Thus, we intended to explore the expression and prognostic relevance of tumor-endogenous PD-1 and its cellular effect through clinical samples and in vitro experiments. We present the following article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1644/rc).
Methods
Patients and tissue samples
This study’s clinical samples were collected from a prospectively maintained data-base (West China Lung Cancer Database, WCLCD), including all the patients operated in the Department of Thoracic Surgery, West China Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of West China Hospital (approval number: 2016-98) and individual consent for this retrospective analysis was waived. We retrospectively collected paraffin specimens of 100 patients who underwent surgery in our department and pathologically diagnosed as NSCLC from July 2008 to June 2009. According to the 8th edition American Joint Committee on Cancer (AJCC) Classification, all cases were histologically evaluated. We collected the clinicopathologic data of these cases, including gender, age, smoke history, tumor size, tumor location, pathological type, pathological TNM stage and adjuvant therapy. Follow-up data has been updated to January 2020. Of all these patients, 32 patients have received adjuvant chemotherapy or radiation after surgery. No patients have received immunotherapy. Additionally, we collected tumor samples from 4 patients who underwent surgery in our department and intraoperative frozen section diagnosed as adenocarcinoma in September 2018 for flow cytometry analysis.
Cell preparation and flow cytometry
We minced the collected tissue into 1–2 mm pieces using scissors, and then dispersed cells by gentle pipetting and filtered through a sterilized cell strainer for 2 times and collected the cell suspension. Centrifuge the suspension 300 g for 10 minutes at 4 °C and then we collected the cell pellet. We washed the pellet 3 times with cold PBS, and then resuspended with 100–200 μL volume of PBS. Finally, we added 1 μg CD45 monoclonal antibody (Innovative Research Cat# MHCD45065, RRID:AB_1475933), 1 μg PD-1 monoclonal antibody (Thermo Fisher Scientific Cat# 25-9985-82, RRID:AB_10853805) into the cell suspension, respectively. Their IgG antibodies were used for the negative control. All samples were incubated for 20 minutes at room temperature and analyzed with CytoFLEX Flow Cytometer (Beckman Coulter, Inc., Brea, CA, USA), protected from light.
Immunohistochemical (IHC)
We deparaffinized and rehydrated the tissue sections with 95%, 70% and 50% alcohols respectively for 3 minutes. We conducted antigen retrieval using 10 mM citrate buffer at 95 °C for 10 minutes. We then incubated and blocked the tissue sections with 3% H2O2 for 15 min and 10% fetal bovine serum (FBS) for 1 hour, respectively. We then incubated the tissue sections with anti-PD-1 antibody (1:200; R and D Systems Cat# AF1086, RRID:AB_354588) at 4 °C overnight and anti-rabbit secondary antibody (1:1,000, ZSGB-Bio Cat# PV-6001, RRID:AB_2864333) at room temperature for 30 minutes, respectively. After that, we stained the sections with DAB substrate solution and substrate-chromogen solution for 2 minutes, and hematoxylin for 1 minute, respectively. Two researchers (Chuanfen Zhang and Fanyi Gan) assessed the IHC results in a blinded way under microscopy. According to the expression of tumor-endogenous PD-1, we classified the immunostained results into positivity and negativity. Positivity was defined as tumor-endogenous PD-1 was observed in any one field by any one of the researchers during analysis. Negativity was defined as no tumor-endogenous PD-1 expression could be observed in all fields by two researchers.
Western Blot
We lysed the cultured tumor cells using RIPA lysis and extraction buffer (Solarbio, Beijing, China) with PMSF (Solarbio, Beijing, China). We measured the cell lysis buffer with the BCA protein assay kit (Solarbio, Beijing, China) and separated 50 μg protein samples in the 10% SDS-polyacrylamide gels. We then transferred the proteins onto polyvinylidene fluoride (PVDF) membrane (wet transfer, 90 minutes, 100 V). We then blocked the membrane with 5% non-fat milk at room temperature for 45 minutes. Anti-PD-1 Polyclonal IgG (1:1,000; R and D Systems Cat# AF1086, RRID:AB_354588) was used as primary antibody, and incubated at 4 °C overnight. An Anti-Goat IgG (1:10,000; LI-COR Biosciences Cat# 926-32214, RRID:AB_621846) was used as the secondary antibody, and incubated at room temperature for 30 minutes protected from light. Finally, we visualized the fluorescent blots with Odyssey Imager (Li-COR, NE, USA). We incubated the membrane with an anti-β-actin (1:1,000, ZSGB-Bio Cat# TA-09, RRID:AB_2636897) monoclonal antibody as the loading control.
Establishment of PD-1 overexpression cell lines
We purchased Pdcd1 overexpression lentivirus from Genechem Company, Ltd. (Shanghai, China). 2×105 cells were seeded in a 6-well plate for 24 hours. Change medium and add 1 μL Pdcd1 lentivirus, and add the vector for negative control. The medium containing lentivirus was changed after 24 hours. Add puromycin (1.0 μg/mL, ThermoFisher, MA, USA, #A1113803) to select cells for about 10–14 days. Change the medium containing puromycin every 3 days and passage the cells when they reached confluency. Finally, HCC827PD-1-OE and H1975 PD-1-OE stable cell lines were established.
Cell viability assay
Cells were seeded at 5×103 cells per well in 96-well plates with complete medium, incubated overnight in a humidified incubator. Added 100 μL complete medium including 10 μL of CCK-8 reagent (#HY-K0301, MedChemExpress, NJ, USA) into each well and incubated for 1 hour. The optical density (OD) value of 450 nm was analyzed on a Biotek ELx800 plate reader (BioTek Instruments, Inc., VT, USA). The results of cell viability were expressed as Relative Growth Rate [1].
OD0 is defined as the OD value 8 hours after seeded and stained with CCK-8 reagent.
ODt is defined as the OD value 24, 48, 72, 96 hours after the first analysis with CCK-8 reagent.
Plate clone formation assays
Cells were plated (0.5–1)×103 cells per well in six-well plates with medium containing 10% FBS. Incubate the cells in the incubator for 2–3 weeks, and add medium containing 10% FBS every 3–4 days. Wash the cells with PBS for 2 times and fix them with 4% paraformaldehyde for 20 minutes. Then, the cells were incubated with 0.1% crystal violet at room temperature for 20 minutes. Finally, discard the crystal violet and wash the cells with PBS 3 times. Count the colonies containing >50–100 cells for analysis.
Scratch assays
Cells were plated in six-well plates with medium containing 10% FBS. Incubation for 3–4 days until the cells reached about 100% confluency. Then, remove the medium and scratches were made in the wells using the sterilized pipette tips. Wash 3 times with PBS until no obvious floating cells or cell fragments could be observed under the microscope. Add medium without FBS. Images were taken for the same labeling sites after 24 and 48 hours with a Nikon Internalight C-HGF1 fluorescence source (Nikon, Mellville, NY, USA).
Statistical analysis
SPSS 23.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8 (GraphPad Inc., La Jolla, CA, USA) were used for statistics. The relationship between tumor-endogenous PD-1 and clinicopathological variables was analyzed by the Pearson chi-square test. Survival was analyzed with Kaplan-Meier survival curve. Univariate and multivariate analyses were performed with the Cox model. The hazard ratio (HR) and a confidence interval of 95% were applied in this analysis. A two-sided P<0.05 was considered statistically significant.
Results
We prepared tumor cell suspensions from 4 patients, operated in our center and intraoperative frozen section diagnosed as adenocarcinoma. Flow cytometry analysis was performed with PD-1 and CD45. The results showed that CD45 (−)/PD-1 (+) cells constitutes 2.08%, 2.42%, 1.79%, 1.21%, respectively. CD45 (−)/PD-1 (+) cells were regarded as the tumor cells endogenously expressing PD-1 and CD45 (+)/PD-1 (+) cells were regarded as the lymphocytes expressing PD-1 (Figure 1A,1B). Then, to investigate the relationship between tumor-endogenous PD-1 and prognosis, we detected the expression of PD-1 by IHC in clinical samples from the WCLCD. The 100 patients of this cohort were all operated between July 2008 and June 2009 in our center and pathologically diagnosed as NSCLC. Of these patients, 34 patients (34%) were positive and 66 (66%) were negative for tumor cell-intrinsic PD-1 (Figure 1C). Immunohistochemical results showed TILs and a small fraction of tumor cells could be positive for PD-1 (Figure 1D). However, the obvious morphological differences between TILs and tumor cells make it easy to clarify the tumor cell-intrinsic PD-1 in the IHC. Correlation of tumor cell-intrinsic PD-1 expression and the clinicopathological characteristics of patients were summarized in Table 1. It showed that the expression of PD-1 was positively correlated with lymph nodes metastasis (OR =3.416, P=0.010), but not with gender, age, smoking history, tumor location and size, pathological type, TNM stage (I vs. II-IV) and adjuvant therapy. The results of PD-1 expression classified by the pathological N stage were showed in Figure 1E.
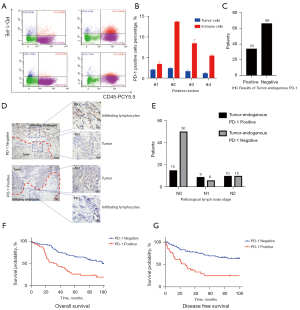
Table 1
| Clinicopathological variables | n | PD-1 expression | χ2 value | P value | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| All patients | 100 | 66 | 34 | ||
| Gender | 0.688 | 0.407 | |||
| Male | 70 | 48 | 22 | ||
| Female | 30 | 18 | 12 | ||
| Age (year) | 0.186 | 0.666 | |||
| ≤60 | 47 | 30 | 17 | ||
| >60 | 53 | 36 | 17 | ||
| Smoke | 0.477 | 0.490 | |||
| No | 43 | 30 | 13 | ||
| Yes | 57 | 36 | 21 | ||
| Tumor location | 2.350 | 0.125 | |||
| Left | 66 | 47 | 19 | ||
| Right | 34 | 19 | 15 | ||
| Tumor size | 0.038 | 0.845 | |||
| ≤3.0 cm | 34 | 22 | 12 | ||
| >3.0 cm | 66 | 44 | 22 | ||
| Pathological type | 0.160 | 0.689 | |||
| Adenocarcinoma | 62 | 40 | 22 | ||
| Squamous carcinoma | 38 | 26 | 12 | ||
| Lymph node metastasis | 6.629 | 0.010 | |||
| Negative | 38 | 31 | 7 | ||
| Positive | 62 | 35 | 27 | ||
| TNM stage | 2.907 | 0.088 | |||
| I | 38 | 29 | 9 | ||
| II-IV | 62 | 37 | 25 | ||
| Adjuvant chemotherapy or radiation | 32 | 22 | 10 | 0.159 | 0.691 |
To explore the prognostic factor for patients with tumor cell-intrinsic PD-1, we collected the survival data from WCLCD and performed univariate and multivariate analysis (Table 2). No patients of the cohort have received immunotherapy ever. Patients with tumor-endogenous PD-1 had significantly worse five-year overall survival (OS) (Figure 1F) and disease-free survival (DFS) (Figure 1G). Multivariate Cox’s regression analysis showed that tumor cell-intrinsic PD-1 expression was an independent prognostic risk factor (HR =3.807, 95% CI: 2.031–7.135, P<0.05*). These results together demonstrated that tumor cell-intrinsic PD-1 could be observed in lung cancer, and it was an independent prognostic risk factor.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| PD-1 expression | 3.902 | 2.135–7.134 | 0.000* | 3.807 | 2.031–7.135 | 0.000* | |
| Positive vs. negative | |||||||
| Gender | 1.061 | 0.562–2.002 | 0.855 | ||||
| Male vs. female | |||||||
| Age (years) | 0.905 | 0.500–1.639 | 0.742 | ||||
| >60 vs. ≤60 | |||||||
| Smoke | 0.81 | 0.448–1.463 | 0.485 | ||||
| Yes vs. no | |||||||
| Tumor location | 1.419 | 0.772–2.607 | 0.259 | ||||
| Right vs. left | |||||||
| Tumor size (cm) | 0.828 | 0.451–1.519 | 0.541 | 0.608 | |||
| ≥3 vs. <3 | |||||||
| Lymph node metastasis | 2.563 | 1.292–5.084 | 0.007* | 1.166 | 0.216–6.306 | 0.858 | |
| Positive vs. negative | |||||||
| TNM stage | 2.325 | 1.194–4.525 | 0.013* | 2.409 | 0.445–13.059 | 0.308 | |
| II-IV vs. I | |||||||
*, P<0.05; CI, confidence interval.
To explore the expression of PD-1 in lung cancer cell lines and clarify the molecular mechanisms between PD-1 expression and prognosis, in vitro experiments were conducted. PD-1 expression was analyzed in 4 common lung cancer cell lines (A549, H1975, H1299 and HCC827) by western blot and flow cytometry. It showed that all of the above cell lines could be observed PD-1 expression in WB, and the PD-1 expression of H1975 and HCC827 was relatively higher than in A549 and H1299 (Figure 2A). PD-1 was also detected in 4 cell lines in FCM analysis and the statistical results were showed in Figure 2B-2F. To sum up, the tumor-endogenous PD-1 was expressed in a small fraction of lung cancer cells, relatively lower than that in melanoma or liver cancer cells. Together with clinical samples results, we concluded that tumor cell-intrinsic PD-1 was expressed in lung cancer, and it played a crucial role in lung cancer recurrence and metastasis, which was an independent prognostic risk factor.
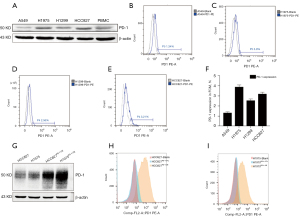
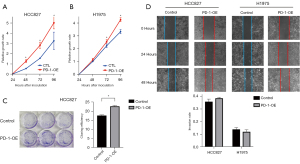
Next, the overexpression of PD-1 was produced in HCC827 and H1975 cells by lentivirus infection (Figure 2G-2I). We investigated the role of PD-1 in tumor proliferation, colony formation and migration. In the CCK-8 proliferation assays, HCC827PD-1-OE and H1975 PD-1-OE exhibited a more vital proliferative ability than control (Figure 3A,3B). Correspondingly, in the culture plate cloning assay, cell-intrinsic PD-1 promoted colony formation of HCC827 cells (P=0.0104) (Figure 3C). However, the difference between PD-1 overexpression and control was not statistically significant in the scratch assays, with the P value was 0.1182 and 0.2946 in HCC827 and H1975 group respectively (Figure 3D).
Discussion
PD-1/PD-L1 axis and its related signaling pathways have become a focus of cancer immunotherapy research over the last decade (17). PD-1 had been considered mainly expressed on the activated T cells and B cells until two studies suggested that PD-1 could be detected in melanoma and liver cancer cells, respectively (15,16). However, whether PD-1 is endogenously expressed in tumor cells of lung cancer has not been reported.
Our results showed that tumor cell-intrinsic PD-1 could be observed in clinical samples and cell lines of NSCLCs. In vitro, it is interesting to note that the expression of PD-1 in H1975 and HCC827 cell lines was stronger than in A549 and H1299 at the protein level. Consistently, it has been reported that the expression level of PD-L1 in H1975 and HCC827 cell lines is also relatively high (18). More than that, PD-1 functions inevitably by interacting with PD-L1. Therefore, we are prone to speculate that the PD-L1 of tumor cells may induce the expression of tumor-derived PD-1 in lung cancer. The expression level of endogenous PD-1 in lung cancer cells in this study is lower than in melanoma cells (15), which could be attributed to the unique immunologic context of different malignancies (19). Studies have classified that tumors may be categorized based on immunologic indicators, such as immunogenicity, tumor mutation load (TMB) and inflammation-related gene expression. Next, researchers integrated these immunologic indicators and developed the “immune score” system in colorectal cancer, and found that the prediction of treatment responsiveness and prognosis was better than traditional TNM staging (20). Lung cancer and melanoma both belong to the high immunogenic tumors with high TMB and expression of inflammation-related genes. Therefore, tumor cell-intrinsic PD-1 being observed in these high immunogenic tumors could be understood. Portraying the immunologic context of different tumors or different individuals with the same tumor is critical to predicting immunotherapy’s efficacy. Tumor-endogenous PD-1 may become a promising evaluation index of tumor immunologic context in the future.
In our results, the tumor-endogenous PD-1 could be observed in 34% of the included patients, and no significant correlation was observed between PD-1 expression and gender, age, smoking history, tumor location, tumor size, pathological type, and TNM staging (I vs. II-IV). However, in the TNM staging (I vs. II-IV), a higher positive rate was observed in stage II-IV than stage I. Due to the limited sample size, no statistically significant difference was observed between Stage I and II-IV. Thus, we need to expand the sample size in a future study. PD-1 expression was correlated with lymph node metastasis (OR =3.416, P=0.010), suggesting that tumor-endogenous PD-1 may be involved in lung cancer progression or metastasis. Consistently, in the Kaplan-Meier survival analysis, patients with tumor-endogenous PD-1 expression showed inferior PFS and OS. Tumor-endogenous PD-1 was an independent prognostic risk factor. For clinicians, these outcomes may indicate that regular postoperative follow-up is critical for these patients with tumor-endogenous PD-1 expression, who have a greater chance of recurrences and metastasis.
IHC results showed that tumor-endogenous PD-1 expression was mainly observed on the cell membrane, but some could be found in the cytoplasm. Studies reported that the PD-1 on the T cells is glycosylated (21). Glycosylation constitutes a common post-translational modification, including N-glycosylation and O-glycosylation, and plays a vital role in tumor proliferation, invasion and metastasis, and angiogenesis (22). Accordingly, we speculate that there are two forms of PD-1 protein in the tumor cells, membrane protein and cytosolic protein, and the PD-1 on the cell membrane has glycosylation modification. After translation, glycosylation modification of PD-1 is required for correctly locating on the cell membrane and exercise biological function. However, the unglycosylated PD-1 still stays in the cytoplasm. Glycosylation modification of tumor-endogenous PD-1 is in our subsequent experiments.
The traditional view assumed that the PD-1/PD-L1 axis inhibited the immune system and induced immune escape. Moreover, blockade of the interaction between PD-1 and PD-L1 could restore or strengthen T-cell activity against tumor cells, thereby preventing cancer progressing and metastasis (12-14). Anti-PD-1 and anti-PD-L1 agents show promising efficacy in NSCLC, especially for those with high PD-L1 expression. Studies on melanoma and hepatocellular carcinoma extended our understanding of PD-1 in the tumor microenvironment and the molecular mechanisms underlying anti-PD-1 therapy (15,16). Consistently, our observations demonstrate that PD-1 was endogenously expressed on the lung cancer cells, expanding the traditional perception of PD-1 in lung cancer.
Some limitations also existed in this study. First, the number of included patients and samples was limited. We concluded only in a non-representative Chinese population of lung cancer patients and four lung cancer cell lines in vitro. Second, PD-1 was also expressed on immune cells in the tumor microenvironment, and we identified tumor-intrinsic PD-1 based on cell morphology under a microscope. Third, WB, FCM and IHC were used to verify tumor-intrinsic PD-1 at the protein level without any experiments at the mRNA level. However, PD-1 functions at the protein level, so we think studies on the protein level prioritize. Moreover, no in vivo experiments verifying the role of tumor-endogenous PD-1 was carried out. Finally, a more in-depth molecular mechanism of tumor-endogenous PD-1 in lung cancer is out of this study’s scope and needs further research.
Although the limitations mentioned above, our research demonstrated for the first time that PD-1 is endogenously expressed on lung cancer cells, and tumor-endogenous PD-1 could serve as an independent prognostic risk factor. Moreover, in vitro experiments showed that PD-1 could enhance the proliferation and clone formation of lung cancer cells. Experiments on glycosylation modification of tumor-endogenous PD-1, related downstream signaling pathways and potential therapeutic targets are needed in the future.
Conclusions
This study confirmed for the first time that PD-1 was endogenously expressed on the cancer cell of NSCLCs. Expression of tumor-endogenous PD-1 was statistically related to lymph node metastasis, and it was an independent prognostic risk factor for patients. In vitro experiments demonstrated that PD-1 could enhance the proliferation and clone formation of lung cancer cell lines. These results explored the role of PD-1 in tumor immune microenvironment and may provide new thinking for immunotherapy in NSCLCs.
Acknowledgments
We thank Prof. Gao Zhang from Duke University for polishing our manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) (No. 81402240 to Senyi Deng), the Science and Technology Plan Project (No. 2018JY0596 to Lin Ma) Sichuan Province, and the Science and Technology Project (No. 21PJ001 to Chenglin Guo) of the Health Planning Committe of Sichuan, China.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1644/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1644/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1644/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of West China Hospital (approval number: 2016-98) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Zhang X, Schwartz JC, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004;20:337-47. [Crossref] [PubMed]
- Nishimura H, Agata Y, Kawasaki A, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol 1996;8:773-80. [Crossref] [PubMed]
- Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765-72. [Crossref] [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Kleffel S, Posch C, Barthel SR, et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015;162:1242-56. [Crossref] [PubMed]
- Li H, Li X, Liu S, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 2017;66:1920-33. [Crossref] [PubMed]
- Constantinidou A, Alifieris C, Trafalis DT. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol Ther 2019;194:84-106. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151-67. [Crossref] [PubMed]
- Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128-39. [Crossref] [PubMed]
- Okada M, Chikuma S, Kondo T, et al. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep 2017;20:1017-28. [Crossref] [PubMed]
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 2015;15:540-55. [Crossref] [PubMed]


