
A EWSR1-CREM-rearranged gastric mesenchymal tumor accompanied by gastritis cystica profunda and with probable benign behavior: a case report
Introduction
Recurrent genomic arrangements represent a significant molecular characteristic in approximately one-third of sarcomas (1). A significant group of the fusions that lack tumor type-specificity are those involving Ewing sarcoma breakpoint region 1 (EWSR1) and genes of the CREB family of transcription factors (CREB, ATF1, and CREM), which have been reported in a wide spectrum of mesenchymal tumors, including clear cell sarcomas (CCSs), CCS-like tumors of the gastrointestinal tract (CCSLGT), angiomatoid fibrous histiocytoma (AFH) with intracranial predilection, pulmonary myxoid sarcoma, and malignant epithelioid neoplasm with predilection for mesothelial-lined cavities, among which only CCSLGT is located in the gastrointestinal (GI) tract (2-4).
Gastritis cystica profunda (GCP) is a rare disease first described in 1972 (5). GCP is characterized by hyperplasia and cystic dilatation of gastric glands that extend into the submucosa (6). The cystic expansion may also occur in the mucosa and/or muscularis propria in some cases, while in others the mucosa may appear normal (6,7). GCP is difficult to diagnose preoperatively owing to nonspecific symptoms and radiographic appearances, and most of the 52 patients reported so far underwent surgical excision. There is currently no evidence of recurrence or metastasis, suggesting a benign course (6). However, GCP was reported to show elevated proliferation and DNA repair and occur in 3% gastric carcinomas (6), and several cases have shown early gastric cancer associated with GCP, including three in which an adenocarcinoma (ADC) was partially within GCP. Therefore, GCP is generally regarded as a precursor of gastric tumor (6,8). There are currently 5 patients reported to have gastric cancer arising from or associated with GCP, all of which ADCs (Table 1) (9-13).
Table 1
| Ref. | Age | Sex | Prior gastric surgery | Symptom | Involved gastric layer(s) | Gastric tumor | GCP IHC | Treatment | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|
| (9) | 63 | M | No | Abdominal pain | Mucosa | ADC | KCNE2, ER | Distal gastrectomy | NR |
| (10) | 55 | M | No | No stomach or intestinal symptoms | Submucosa | ADC | NR | Endoscopic submucosal dissection | AWED, 39 months |
| (11) | 45 | M | No | Epigastric abdominal pain and black stool | Mucosa, submucosa | ADC | NR | Total gastrectomy with esophagojejunostomy, abdominal lymphadenectomy, feeding jejunostomy, and bilateral vagotomies | NR |
| (12) | 51 | M | No | Abdominal pain | Submucosa, muscularis propria, subserosa | ADC | Ki-67, p53, p21WAF1/CIP1 | Total gastrectomy | AWED, 10 years |
| (13) | 83 | F | Gastrojejunostomy 50 years prior | Epigastric discomfort | Mucosa, submucosa | ADC, signet ring cell carcinoma | Ki-67 | Distal gastrectomy, regional lymphadenectomy | AWED, 46 months |
| (14) | 67 | M | No | Melena, ulcerated bleeding | Submucosa, muscularis propria | No tumor observed. GCP lesion mimicked GIST in GI tract bleeding and endoscopic ultrasonography | NR | Surgical resection of the lesion | NR |
| (15) | 61 | M | No | None | Mucosa, submucosa, muscularis propria | No tumor observed. GCP lesion mimicked solid submucosal tumors in radiologic evaluation | CD68 | Laparoscopic wedge resection | AWED. Duration not reported |
GCP, gastritis cystica profunda; IHC, immunohistochemistry; ADC, adenocarcinoma; NR, not reported; AWED, alive with no evidence of disease; GIST, gastrointestinal stromal tumor; GI, gastrointestinal.
Herein, we report a peculiar case that showed an admixture of GCP and EWSR1-CREM-rearranged gastric mesenchymal neoplasm. Given the patient’s disease-free status at 28 months post-surgery, it was more likely that both lesions were benign. For comprehensive characterization of this admixture, we performed multiple analyses on the surgical specimen, including histologic examination, immunohistochemistry (IHC), next-generation sequencing, fluorescence in situ hybridization (FISH), and follow-up phone calls to track survival outcomes. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2331/rc).
Case presentation
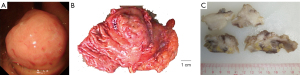
A 69-year-old woman visited our hospital with complaints of dizziness and fatigue for the previous two months. Past medical history was significant for chronic type II diabetes, which was kept under control with Novolin 30R. The patient had not received gastric surgery before. Blood test showed low levels of hemoglobin (87 g/L), mean corpuscular volume, and mean corpuscular hemoglobin. Gastroscopy revealed in the fundus an unencapsulated mass with smooth surface distinctly separated from the surrounding tissues (Figure 1A). Colonoscopy was unremarkable. Laparoscopic partial gastrotomy was subsequently performed in Apr 2019 to remove the polypoid mass, which measured 8 cm in greatest dimension and showed a gray, firm cut surface (Figure 1B,1C). The patient was alive with no evidence of recurrence or metastasis at 28 months follow-up after surgery.
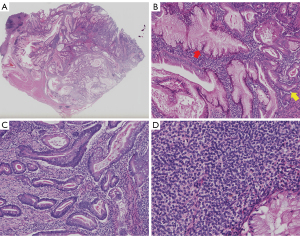
Histologically, the mass showed readily distinguishable epithelial and mesenchymal components. All layers of the gastric wall were invaded, although no lymph node or neural invasion, or tumoral vascular emboli was noted. At the periphery were residual gastric oxyntic gland mucosa (Figure 2A). The epithelial component consisted of MUC5AC-expressing, foveolar-type glands interspersed with MUC6-expressing, pyloric-type glands. Some glands showed metaplastic growth, indicated by the admixture of foveolar-type, pyloric-type, and goblet cells. Most glands were elongated with irregular contour, with some forming cystic structures containing eosinophilic secretory material. The epithelial cells showed focally atypical hyperchromatic nuclei, inconspicuous nucleoli, slightly eosinophilic cytoplasm, and infrequent mitosis (Figure 2B,2C). The mesenchymal component consisted of monomorphic, ovoid-shaped cells often arranged in sheets surrounding the glands. These cells displayed scanty cytoplasm, regular nuclei, and rare mitotic figures (Figure 2D).
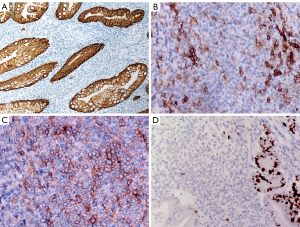
On IHC, the epithelial cells were uniformly positive for cytokeratins and negative for markers of neuroendocrine differentiation (Figure 3A). The mesenchymal cell showed focal positivity for CD10 and CD117 (Figure 3B,3C), focal, patchy positivity for CD56, negativity for cytokeratin, neuroendocrine markers, DOG-1, CD34, SMA, desmin, HMB-45, Melan A, and S-100. Ki-67 index was low in the mesenchymal cells (1%; Figure 3D). Interestingly, genomic profiling with a targeted panel of 520 cancer-related genes (OncoScreen Plus, Burning Rock, Guangzhou, China) revealed an EWSR1-CREM (E15:C7) fusion, which was further confirmed by FISH using both fusion and split‐apart probes (Figure 4). Furthermore, this fused gene appeared to be detectable exclusively in the mesenchymal component.
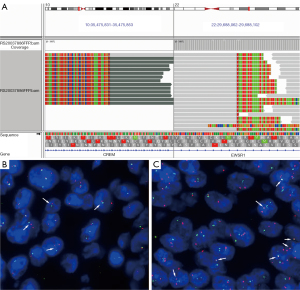
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In summary, we report a gastric fundic mass consisting of readily distinguishable epithelial and mesenchymal components. The epithelial cells showed morphology and immunophenotype consistent with metaplasia and downgrowth of gastric glands into the submucosa, suggesting GCP. The mesenchymal component was comprised of ovoid-shaped cells harboring EWSR1-CREM fusion. Given the absence of lymph node, vascular or neural invasion, low mitotic activity, and disease-free status at 28-month follow-up, the GCP and mesenchymal tumor were likely benign.
A panel of mesenchymal tumors of the GI tract was considered for the mesenchymal component. CCSLGT was considered based on the anatomic site and presence of EWSR1-CREM fusion. However, CCSLGT is a malignant entity that occurs mostly in young adults. In a series of 3 CCSLGT cases that harbored EWSR1-CREB family fusion, the patient age ranged 24–29, and one developed lung metastasis and died after 2 years (16). Also, all three patients displayed patchy positivity for S-100 and negativity for CD117 on IHC, further excluding a CCSLGT diagnosis (17). The immunostaining results in this case agreed with gastrointestinal stromal tumor (GIST) on CD117 positivity and S-100 negativity. However, CD117 negativity occurs in up to 18% of gastric GISTs, making it a less specific marker (17). Furthermore, DOG-1 immunoreactivity in reported in 87–97% GISTs (18) and CD34 in 60–70% (17), both of which were lacking in this case. Morphologically, most GISTs are composed of either short fascicles of spindle cells or round, epithelioid cells arranged in nests, which contrasts with the ovoid cells arranged in sheets in this case. Other tumor types, such as smooth muscle neoplasms and tumors with fibroblastic/myofibroblastic, neural, or melanocytic differentiation, were excluded by respective negative immunostaining for SMA, desmin, neuroendocrine markers, and melanocytic markers in this case (17). Recent years have also seen an explosion in the histological spectrum of mesenchymal tumors with EWSR1/FUS-CREB family fusions (2-4,19). Shibayama et al. (19) reported 8 cytokeratin-positive intra-abdominal malignancies harboring EWSR1/FUS-CREB fusions. Among these cases, one was strikingly resemblant to ours in that a gastric submucosal mesenchymal neoplasm was admixed with dilated epithelial structures that invaginated from the mucosa. EWSR1-CREB fusion was detected with FISH, and the tumor also showed low mitotic activity. There were, however, peripheral lymphoid cuffing and prominent hemorrhagic pseudoangiomatous spaces suggested of AFH. In addition to cytokeratin positivity, the tumor also showed other discrepancies such as epithelioid cytomorphology, diffuse SMA positivity, and possibly a more aggressive clinical course. Interestingly, 2 subsequent specimens resected in the abdominal cavity at recurrence displayed similar histology but without the epithelial inclusions, suggesting the GCP-like structure as a completely independent or stomach-specific phenomenon. However, conclusive elucidation awaits further research.
In addition to the case series by Shibyama and colleagues, Argani et al. (4) also described 13 EWSR1/FUS-CREB-rearranged malignant epithelioid neoplasms, most of which located intra-abdominally or expressing epithelial markers cytokeratin and/or EMA. An interesting finding was an EWSR1-CREM fusion that attaches CREM exon 7 to EWSR1 exon 15, the same composition as with this case. One of the EWSR1-CREM-rearranged tumors was intra-abdominal and inseparable from gastric fundus, although it was not specified which breakpoints were detected. Since EWSR1 exon 15 is a highly rare breakpoint, this similarity may reflect shared molecular basis between these cases and ours despite immunohistochemical and clinical differences. As new EWSR1/FUS-CREB-rearranged mesenchymal tumors are detected and new classes proposed at a rapid pace, it is possible that this case falls into one of the recorded classes, although more insights into the relationship between the pathogenesis of GCP and the mesenchymal neoplasm are warranted.
GCP was previously associated with a history of gastric surgery, although a recent review found GCP occurring approximately equal percentages of patients with (52%) and without surgery (48%) (6). However, GCP was observed in animals after gastrectomies or predisposition to H. pylori infection, suggesting association with insult to the gastric mucosa (6,10). Highly suspected as precancerous, GCP was simultaneously found together with gastric ADC in 5 cases (9-13). There therefore appears to be an association among mucosal insult, GCP, and neoplastic growth. In this case, it is unknown whether GCP and mesenchymal neoplasm developed independently from one other or in concert, although considering the rarity of both entities and the precancerous nature of GCP, the two lesions may be pathogenetically connected. On the other hand, evidence is still amassing as to the association between surgical resection and GCP (20), and a recent study observed GCP and ADC 50 years after gastrojejunostomy at the anastomotic site (13). It is therefore advisable to monitor disease recurrence on a regular basis.
To our knowledge, this is the first to report an EWSR1-CREM fusion in a gastric mesenchymal tumor with accompanying GCP. However, due to its anecdotal nature, this case is limited answering the questions it raises. First, it remains to be elucidated whether the GCP and mesenchymal tumor were associated in etiology. Additionally, it is of interest to clarify whether this admixture represents a novel of class of gastric mesenchymal tumors and, if yes, questions such as its epidemiology, symptoms, prognosis, and treatments would warrant further investigations. A review of previous cases may be in order, since there have been isolated reports of GCP mimicking GIST (14) or solid submucosal tumor (15) (Table 1).
Acknowledgments
We would like to thank the patient and her family for their support. We are also grateful to Xiao Zou and Min Li from Burning Rock Biotech for technical assistance.
Funding: This work was supported by National Natural Science Foundation of China (grant No. 81802719), Medical Scientific Research Foundation of Guangdong Province, China (No. A2017433), and Research Grant of Guangdong Provincial People’s Hospital (No. Y02108141).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2331/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2331/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taylor BS, Barretina J, Maki RG, et al. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer 2011;11:541-57. [Crossref] [PubMed]
- Yoshida A, Wakai S, Ryo E, et al. Expanding the Phenotypic Spectrum of Mesenchymal Tumors Harboring the EWSR1-CREM Fusion. Am J Surg Pathol 2019;43:1622-30. [Crossref] [PubMed]
- Segawa K, Sugita S, Aoyama T, et al. Detection of specific gene rearrangements by fluorescence in situ hybridization in 16 cases of clear cell sarcoma of soft tissue and 6 cases of clear cell sarcoma-like gastrointestinal tumor. Diagn Pathol 2018;13:73. [Crossref] [PubMed]
- Argani P, Harvey I, Nielsen GP, et al. EWSR1/FUS-CREB fusions define a distinctive malignant epithelioid neoplasm with predilection for mesothelial-lined cavities. Mod Pathol 2020;33:2233-43. [Crossref] [PubMed]
- Littler ER, Gleibermann E. Gastritis cystica polyposa. (Gastric mucosal prolapse at gastroenterostomy site, with cystic and infiltrative epithelial hyperplasia). Cancer 1972;29:205-9. [Crossref] [PubMed]
- Du Y, Zhang W, Ma Y, et al. Gastritis cystica profunda: a case report and literature review. Ann Palliat Med 2020;9:3668-77. [Crossref] [PubMed]
- Yu XF, Guo LW, Chen ST, et al. Gastritis cystica profunda in a previously unoperated stomach: a case report. World J Gastroenterol 2015;21:3759-62. [Crossref] [PubMed]
- Li C, Song S, Wu G, et al. Gastritis cystica profunda: clinical and pathologic study of seven cases and review of literature. Int J Clin Exp Pathol 2021;14:261-6. [PubMed]
- Kuwahara N, Kitazawa R, Fujiishi K, et al. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J Gastroenterol 2013;19:1314-7. [Crossref] [PubMed]
- Ogasawara N, Noda H, Kondo Y, et al. A case of early gastric cancer arising from gastritis cystica profunda treated by endoscopic submucosal dissection. Case Rep Gastroenterol 2014;8:270-5. [Crossref] [PubMed]
- Wahi JE, Pagacz M, Ben-David K. Gastric Adenocarcinoma Arising in a Background of Gastritis Cystica Profunda. J Gastrointest Surg 2020;24:2387-8. [Crossref] [PubMed]
- Mitomi H, Iwabuchi K, Amemiya A, et al. Immunohistochemical analysis of a case of gastritis cystica profunda associated with carcinoma development. Scand J Gastroenterol 1998;33:1226-9. [Crossref] [PubMed]
- Namikawa T, Kawanishi Y, Fujisawa K, et al. Gastric adenocarcinoma at the anastomotic site 50 years after gastrojejunostomy: A case report. Mol Clin Oncol 2017;7:249-51. [Crossref] [PubMed]
- Carvalho JR, Quadros AC, Meireles L, et al. Gastritis cystica profunda mimicking a GIST - A diagnostic challenge. Gastroenterol Hepatol 2018;41:448-50. [Crossref] [PubMed]
- Noh SJ, Kim KM, Jang KY. Gastritis cystica profunda with predominant histiocytic reaction mimicking solid submucosal tumor. Turk J Gastroenterol 2020;31:726-8. [Crossref] [PubMed]
- Sonai MK, Rastogi S, Madhusudhan KS, et al. Clear cell sarcoma like tumor of gastrointestinal tract: Experience of three cases and review of literature. Indian J Pathol Microbiol 2020;63:90-5. [Crossref] [PubMed]
- Sbaraglia M, Businello G, Bellan E, et al. Mesenchymal tumours of the gastrointestinal tract. Pathologica 2021;113:230-51. [Crossref] [PubMed]
- Charville GW, Longacre TA. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv Anat Pathol 2017;24:336-53. [Crossref] [PubMed]
- Shibayama T, Shimoi T, Mori T, et al. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. Am J Surg Pathol 2022;46:134-46. [Crossref] [PubMed]
- Cao S, Zou T, Sun Q, et al. Safety and long-term outcomes of early gastric cardiac cancer treated with endoscopic submucosal dissection in 499 Chinese patients. Therap Adv Gastroenterol 2020;13:1756284820966929. [Crossref] [PubMed]


