
A modified method for Billroth-II gastrojejunostomy after laparoscopy-assisted distal gastrectomy
Introduction
Laparoscopy-assisted distal gastrectomy (LADG) is widely performed for gastric cancer in Eastern countries (1-4). Following LADG, reconstruction methods mainly include Billroth-I, Billroth-II and Roux-en-Y. There are merits and demerits to each of them. Billroth-I is simple and enables more physiological processing of food postoperatively. However, only when the remnant stomach is large enough can this procedure be safely performed. It is widely used in Japan and Korea, where tumors are usually detected at an early stage. In other parts of the world, gastric cancers are usually diagnosed at an advanced stage. Therefore, Billroth-II and Roux-en-Y, which enable wider stomach resection without increasing anastomotic tension, are more favored (5). Compared to Roux-en-Y, Billroth-II is simpler, requires only one anastomosis, and takes less time. However, Billroth-II has also been criticized for increased reflux gastritis, as well as afferent loop syndrome (6,7). Many surgeons tend to add a Braun anastomosis (a side-to-side anastomosis between afferent and efferent loops distal to the gastrojejunostomy site) to reduce postoperative complications (8,9). However, this approach inevitably increases the complexity of the operation, and some obese patients may need the surgical incision extended to complete the procedure.
In Billroth-II with Braun anastomosis, there are two separate anastomoses: a proximal gastrojejunal anastomosis and a distal jejunojejunal anastomosis. The latter reduces afferent loop syndrome and reflux gastritis by diverting a substantial amount of bile from the duodenum to the efferent limb. We hypothesized that these two anastomoses could be integrated as one procedure without compromising their functions. To achieve this aim, we developed a modified Billroth-II gastrojejunostomy technique, referred to as “pant-shaped anastomosis”, which can effectively reduce the incidence of reflux gastritis and prevent afferent loop syndrome.
This study describes the surgical method for this novel technique and analyzes the surgical outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2220/rc).
Methods
Patients
Between June 2018 and June 2019, 96 patients with distal gastric cancer underwent LADG with pant-shaped anastomosis at The First Affiliated Hospital of Wannan Medical College. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Yijishan Hospital of Wannan Medical College (No. YJSYY20180502) and individual consent for this retrospective analysis was waived. The operations were performed by the same experienced surgeon team (led by Dr. Shi). Medical records of these patients were reviewed retrospectively, including sex, age, body mass index (BMI), previous abdominal surgery history, American Society of Anesthesiologists (ASA) score, tumor size, tumor-node-metastasis (TNM) stage, total harvested lymph node number, metastatic lymph node number, time for the total operation, time for digestive tract reconstruction, intraoperative blood loss, number of cases converted to open laparotomy, upper abdominal incision length, time to first flatus, time to first oral intake, and length of hospital stay.
Early complications refer to any unintended event occurring within 30 days of surgery, whereas late complications refer to those occurring within 31 days to 12 months after surgery. Anastomosis-related complications refer to events occurring near the pant-shaped anastomosis, including extraluminal bleeding, intraluminal bleeding, anastomosis leakage, afferent obstruction, duodenal stump leakage, internal herniation, and pancreatitis. They were diagnosed by computed tomography, upper gastrointestinal radiology, esophagogastroduodenoscopy findings, as well as clinical signs and symptoms. The Clavien-Dindo classification system was used to classify the severity of the complications.
Patients were followed up with regular outpatient visits at 1, 3, 6, 12, 18, and 24 months postoperatively, and gastroscopy was routinely performed at 12 and 24 months. The patients were specifically asked about diarrhea, early dumping, appetite, weight gain or loss, heartburn, and epigastric pain, as well as if they needed to take antiulcer or antireflux medications. The Visick score was used to evaluate these subjective surgical outcomes (10), as follows: Visick I, no symptoms (complete satisfaction); Visick II, infrequent or minor symptoms (patients with mild symptoms not requiring medical treatment); Visick III, significant symptoms (patients requiring medical treatment for their symptoms); and Visick IV, severe intractable symptoms (patients having severe symptoms despite drug treatment, therefore needing an operation). Postoperative reflux gastritis was evaluated by gastroscopic images and defined as superficial gastritis exhibiting findings such as redness, edema and bleeding tendency (11). The endoscopic severity of reflux gastritis was assessed in four grades as follows: grade 0, no redness; grade 1, mild redness around the anastomosed region; grade 2, comb-shaped marked redness in the greater curvature on the oral side of the anastomosed area; and grade 3, diffuse severe redness and marked edema.
Surgical technique
General procedure
After satisfactory induction of general anesthesia, the patient was placed in the supine position with legs slightly apart. Through a small infraumbilical incision, a 12-mm trocar was inserted, and pneumoperitoneum was achieved. A laparoscopic camera was then introduced. Two 5-mm assistant ports (lower and upper) were inserted on the right, and two 12-mm operator ports were inserted on the left under close surveillance. Following an inspection of the peritoneal cavity, distal gastrectomy was performed with lymphadenectomy according to the Japanese Gastric Cancer Treatment Guidelines (12). After mobilization of the stomach, the duodenum bulb was transected just below the pyloric ring intracorporeally using endoscopic linear staplers. Then, a 5-cm long midline incision was made in the upper abdomen, and the fully mobilized stomach was removed from the abdominal cavity. Subsequent digestive tract reconstruction was performed extracorporeally.
Pant-shaped anastomosis procedure
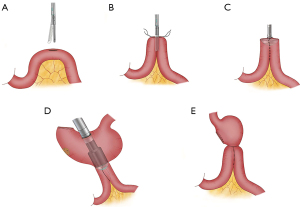
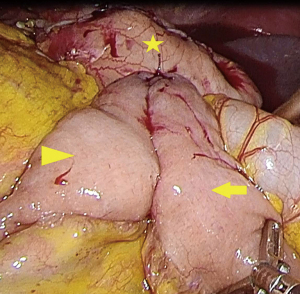
The jejunum was lifted in front of the transverse colon through the middle incision of the upper abdomen. A 1.0 cm longitudinal incision was made on the antimesenteric border of the proximal jejunum, 25–30 cm distal to the ligament of Treitz (Figure 1A). The two arms of a 60 mm linear stapler with white cartilage were inserted into the afferent loop and efferent loop. By firing the stapler, a side-to-side enteroenteric anastomosis between the loops was created (Figure 1B). Then, the anvil head of a 29-mm circular stapler was inserted into the common opening of the afferent-efferent loops (Figure 1C). Then, an incision was made on the anterior wall of the stomach, and a 29-mm circular stapler gun was inserted. Next, the anvil was docked onto the end of the stapler gun, and the posterior wall of the remnant stomach was anastomosed to the common opening of the afferent-efferent loop (Figure 1D). Finally, the gastric stump was closed with linear staplers (Figures 1E,2).
Statistical analysis
Continuous variables with a normal distribution are expressed as the mean ± standard deviation; otherwise, they are presented as the median (interquartile range). Count data are expressed as cases/percentages (n/%). SPSS 20.0 was used for data processing and statistical analysis.
Results
Patient’s characteristics
The patients’ demographic and clinical characteristics are described in Table 1. A total of 96 patients with a mean age of 63.3±11.5 years, including 64 male patients and 32 female patients, were enrolled. The mean BMI was 23.1±3.5 kg/m2. Fifteen (15.6%) patients had a previous abdominal surgery history. Regarding the ASA score, 52.1% of patients were ASA 1, 42.7% were ASA 2, and 5.2% were ASA 3. The mean tumor size was 2.2±1.3 cm. The mean number of harvested lymph nodes was 22.5±8.3, while the mean number of metastatic lymph nodes was 3.1±3.8. There were 12 (12.5%), 45 (46.9%) and 39 (40.6%) patients with stage I, stage II and stage III cancer, respectively.
Table 1
| Variable | Value |
|---|---|
| Age (years) | 63.3±11.5 |
| Sex (M/F) | 64/32 |
| BMI (kg/m2) | 23.1±3.5 |
| Previous abdominal surgery history | 15 (15.6%) |
| ASA score, n (%) | |
| 1 | 50 (52.1) |
| 2 | 41 (42.7) |
| 3 | 5 (5.2) |
| Tumor size | 2.2±1.3 |
| Number of harvested lymph nodes | 22.5±8.3 |
| Number of metastatic lymph nodes | 3.1±3.8 |
| TNM stage, n (%) | |
| I | 12 (12.5) |
| II | 45 (46.9) |
| III | 39 (40.6) |
Values are presented as mean ± standard deviation or number (%). BMI, body mass index; ASA, American Society of Anesthesiologists; TNM, tumor-node-metastasis.
Surgical outcomes
As shown in Table 2, all procedures were successfully completed without conversion to open laparotomy. The mean operative time was 155.8±47.5 min, and the digestive reconstruction time was 22±2.5 min. The mean intraoperative blood loss was 47.2±22.8 mL. The mean incision length was 5.3±1.78 cm. The mean time to postoperative first flatus was 3.5 days. The mean time to intake of an oral semiliquid diet was 5.5 days. The average postoperative hospital stay was 8.2 days.
Table 2
| Variable | Value |
|---|---|
| Total operation time (min) | 155.8±47.5 |
| Digestive tract reconstruction time (min) | 22±2.5 |
| Conversion to laparotomy | 0 |
| Intraoperative blood loss (mL) | 47.2±22.8 |
| Upper abdominal incision length (cm) | 5.3±1.78 |
| Time to first flatus (days) | 3.5±0.6 |
| Time to first oral intake (days) | 5.4±0.5 |
| Length of hospital stay (days) | 8.7±1.5 |
Table 3 shows the postoperative complications, postoperative symptoms and endoscopic findings in patients who underwent LADG with pant-shaped anastomosis. One patient developed duodenal stump leakage on the 5th day after the operation, and no patients developed extraluminal bleeding, intraluminal bleeding, anastomosis leakage, afferent obstruction, internal herniation or pancreatitis. Postoperative symptoms were collected in 79 patients. Among them, 60 patients had no symptoms, and 55 patients gained weight. There were 9, 3, 3, 2, and 3 cases of diarrhea, early dumping, appetite loss, heartburn, and epigastric pain, respectively. Visick scores for grades I, II, and III were 75.9%, 14.0%, and 10.1%, respectively. Regarding the severity of reflux gastritis, 6.1% (4/66) had endoscopic grade 0, 56.1% (37/66) had grade 1, 21.2% (14/66) had grade 2, and 16.7% (11/66) had grade 3.
Table 3
| Complications | Value |
|---|---|
| Extraluminal bleeding | 0 |
| Intraluminal bleeding | 0 |
| Anastomosis leakage | 0 |
| Afferent obstruction | 0 |
| Duodenal stump leakage | 1 |
| Internal herniation | 0 |
| Pancreatitis | 0 |
Discussion
In contrast to that in Japan and South Korea, the proportion of advanced gastric cancer in China is still high (13). Billroth-II and Roux-en-Y procedures are more popular for distal gastric cancer, as the stomach can be resected sufficiently without increasing anastomotic tension (14). However, both methods have drawbacks. Billroth-II yields a higher incidence of bile reflux and gastritis (15). In addition, afferent loop syndrome, originating from mechanical obstruction at the anastomosis itself or a point near the anastomosis, is sometimes found, especially in antecolic Billroth-II reconstruction (7). Roux-en-Y can prevent alkaline reflux gastritis, but it is more complicated, and Roux leg stasis and internal hernia are possible complications (16,17).
In 1892, Braun first proposed adding a side-to-side jejunojejunal anastomosis (Braun anastomosis) in addition to gastrojejunal anastomosis to reduce the incidence of bile reflux and afferent loop obstruction (18). However, this approach did not receive as much attention as Roux-en-Y reconstruction in open distal gastrectomy. Due to the difficulty of the Roux-en-Y operation in laparoscopic gastric cancer surgery, Billroth-II with Braun anastomosis has recently gained more popularity. Zang et al. reported that Billroth-II with Braun anastomosis after totally laparoscopic distal gastrectomy can effectively diminish obstruction, twist, protrusion and edema, and allow food to pass through easier (19). Cui et al. reported that Billroth-II with Braun anastomosis is a better alternative to Roux-en-Y reconstruction for laparoscopic distal gastrectomy (20).
In our department, we prefer Billroth-II with Braun anastomosis for LADG. However, sometimes this is still not easy to perform since the incision of laparoscopic surgery is relatively small. If a patient has obesity or a short mesentery, the incision usually needs to be extended before Braun anastomoses can be performed. To some extent, this is contrary to the original intention of minimally invasive laparoscopic surgery.
Here, we describe a modified Billroth-II anastomosis. It first constructs a side-to-side anastomosis between the afferent loop and efferent loop by using a linear stapler and then anastomoses the remnant stomach with the common opening of the afferent-efferent loop by using a circular stapler. Its appearance resembles a pair of pants, so we named it “pant-shaped anastomosis”. According to our hypothesis, pant-shaped anastomosis has the following advantages. First, pant-shaped anastomosis adds a spacious channel between the afferent and efferent loops. Duodenal fluid can enter the efferent loop directly without passing through the stomach, which effectively reduces the pressure in the duodenum. Second, the anastomosis can act like a pouch, housing pancreatic fluid, decreasing enzyme stimulation of the residual stomach, and thereby reducing reflux gastritis, reflux esophagitis, gastroparesis, and anastomotic edema. Furthermore, compared with Billroth-II with Braun anastomosis or Roux-en-Y, pant-shaped anastomosis is easier to perform. Unless anastomotic bleeding occurs, additional hand suturing is not required.
Through preliminary clinical practice involving 96 patients, we found that pant anastomosis is indeed safe and easy to perform. The mean total operative time was 155.8 min, with an average of 22 min required for digestive tract reconstruction. The mean incision length was 5.3 cm. Our follow-up results showed that there were no cases of extraluminal bleeding, intraluminal bleeding, anastomotic leakage, afferent obstruction, internal herniation or pancreatitis. Only one patient developed duodenal stump leakage on the 5th day after the operation, so the rate of pant-shaped anastomosis-related severe complications was 1.5% (1/66). The Visick score showed that the proportion of patients who felt significant reflux was not high (Visick III–IV 10.1%). This result is similar to that previously reported for Roux-en-Y (21). In total, 62.6% of patients exhibited endoscopic reflux gastritis of grade 1 or less, and this percentage was higher than that for Billroth-I or Billroth-II (11).
Our study has several limitations. First, it was a retrospective single-center single-arm study. Second, anastomosis-related complications, such as bleeding, leakage, and afferent obstruction, are rare; therefore, the number of patients included in this study was not large enough to properly evaluate the incidence of complications. Additionally, some postoperative complications may occur 3–5 or even 10 years after the operation, indicating that our follow-up time was comparatively short. Therefore, more high-quality prospective large-sample controlled studies and long-term follow-up are needed to confirm the effect of pant-shaped anastomosis. However, despite these limitations, our study offers a new idea for gastrointestinal reconstruction and the preliminary experience of our team.
In conclusion, pant-shaped anastomosis is a safe and simple procedure. It is a feasible option to reduce afferent obstruction after LADG in patients with distal gastric cancer.
Acknowledgments
Funding: This study was supported by grants from the Research Projects of Anhui Health Committee (AHWJ2021b109) and the “Peak” Training Program for Scientific Research of Yijishan Hospital, Wannan Medical College (KPF 2019017).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2220/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2220/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2220/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Yijishan Hospital of Wannan Medical College (No. YJSYY20180502) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- He Z, Zang L. Reconstruction after laparoscopic assisted distal gastrectomy: technical tips and pitfalls. Transl Gastroenterol Hepatol 2017;2:66. [Crossref] [PubMed]
- Nakamura K, Katai H, Mizusawa J, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol 2013;43:324-7. [Crossref] [PubMed]
- Kim HH, Han SU, Kim MC, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage i gastric cancer: the klass-01 randomized clinical trial. JAMA Oncol 2019;5:506-13. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the class-01 randomized clinical trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- So JB, Rao J, Wong AS, et al. Roux-en-Y or Billroth ii reconstruction after radical distal gastrectomy for gastric cancer: a multicenter randomized controlled trial. Ann Surg 2018;267:236-42. [Crossref] [PubMed]
- Kang KC, Cho GS, Han SU, et al. Comparison of Billroth I and Billroth II reconstructions after laparoscopy-assisted distal gastrectomy: a retrospective analysis of large-scale multicenter results from Korea. Surg Endosc 2011;25:1953-61. [Crossref] [PubMed]
- Grotewiel RK, Cindass R. Afferent loop syndrome. Bethesda: StatPearls Publishing LLC, 2021.
- Wang F, Zu HL, Jiang H, et al. Clinical investigation of combined Billroth II with Braun anastomosis for patients with gastric cancer. Hepatogastroenterology 2014;61:1812-6. [PubMed]
- Li S, Zang L. Research advance in Billroth II with Braun anastomosis after distal gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:956-60. [PubMed]
- Rijnhart-De Jong HG, Draaisma WA, Smout AJ, et al. The Visick score: a good measure for the overall effect of antireflux surgery? Scand J Gastroenterol 2008;43:787-93. [Crossref] [PubMed]
- Abe H, Murakami K, Satoh S, et al. Influence of bile reflux and Helicobacter pylori infection on gastritis in the remnant gastric mucosa after distal gastrectomy. J Gastroenterol 2005;40:563-9. [Crossref] [PubMed]
- Japanese gastric cancer association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Wang FH, Shen L, Li J, et al. The chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. [Crossref] [PubMed]
- Yang K, Hu JK. Gastric cancer treatment: similarity and difference between China and Korea. Transl Gastroenterol Hepatol 2017;2:36. [Crossref] [PubMed]
- Ma Y, Li F, Zhou X, et al. Four reconstruction methods after laparoscopic distal gastrectomy: A systematic review and network meta-analysis. Medicine (Baltimore) 2019;98:e18381. [Crossref] [PubMed]
- Park YS, Shin DJ, Son SY, et al. Roux stasis syndrome and gastric food stasis after laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction in gastric cancer patients: a propensity score matching analysis. World J Surg 2018;42:4022-32. [Crossref] [PubMed]
- Kojima K, Inokuchi M, Kato K, et al. Petersen's hernia after laparoscopic distal gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer 2014;17:146-51. [Crossref] [PubMed]
- Beger HG, Gansauge F. Master of surgery in archiv für klinische chirurgie. Langenbecks Arch Surg 2010;395:17-21. [Crossref] [PubMed]
- Zang W, Liu W, Chen S, et al. B-II reconstruction with Braun’s anastomosis after totally laparoscopic distal gastrectomy with D2 lymph node dissection for advanced gastric cancer. Ann Laparosc Endosc Surg 2016;1. [Crossref]
- Cui LH, Son SY, Shin HJ, et al. Billroth II with Braun enteroenterostomy is a good alternative reconstruction to Roux-en-Y gastrojejunostomy in laparoscopic distal gastrectomy. Gastroenterol Res Pract 2017;2017:1803851. [Crossref] [PubMed]
- Rieu PN, Jansen JB, Biemond I, et al. Short-term results of gastrectomy with Roux-en-Y or Billroth II anastomosis for peptic ulcer. A prospective comparative study. Hepatogastroenterology 1992;39:22-6. [PubMed]


