
Analysis on diagnosis and treatments of 16 cases of extracranial malignant rhabdoid tumor in children
Introduction
Malignant rhabdoid tumor (MRT) is a rare, highly aggressive malignancy that primarily affects infants and young children. It mostly occurs in the kidneys, soft tissues, and central nervous system, with occasional reports in adults (1,2). The incidence of MRT is estimated at 0.6 per million (3). The kidney is the most common site of MRT in children, thus early treatment is often mistaken for Wilms tumor (4). Beckwith et al. (5) first described extracranial rhabdoid tumors as a distinct pathological entity in 1978. In 1981, Haas et al. (6) recognized rhabdoid tumor of the kidney as a separate tumor rather than a variant of Wilms tumor, and introduced the term rhabdoid because of the tumor cells close histological resemblance to rhabdomyoblasts. The median age of onset for patients with MRT of the kidney (MRTK) and extrarenal extracranial MRT (EERT) ranges from 11 to 18 months and 5-year survival rates have been reported to range from 17% to 36% (3,7-9). The time for progression is usually short and the patients who relapse generally do not survive (10). MRT that occurs in the central nervous system is also called atypical teratoid /rhabdoid tumor (AT/RT), and its prognosis is the worst among MRTK, EERT and AT/RT. It has been reported that most children died within 1 year after diagnosis (11). This study conducted a retrospective study on 16 children diagnosed with MRT in the Children’s Hospital of Fudan University to explore their clinical characteristics and prognosis, so as to promote the understanding of MRT. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2548/rc).
Methods
Patient screening, diagnosis, and treatment
Patients who underwent biopsy or surgical resection in the Children’s Hospital of Fudan University were screened from January 2011 to July 2021, diagnosed by pathologists as MRTs based on the characteristics of rhabdoid tumors (12), and excluded cranial atypical teratoid/rhabdoid tumor (AT/RT). In addition, because some of the malignant transformation of the donor kidney or the proper kidney occurred in MRTK (13), patients with a history of kidney transplantation were excluded.
The gender, age, clinical manifestations, location and size of tumor, metastatic lesions, immunohistochemistry, treatments, survival time, and prognosis of the patients who met the inclusion and exclusion criteria were screened. Since some children in the MRTK group underwent preoperative chemotherapy, the tumor size was recorded as the initial size and the “preoperative size after chemotherapy”. Tumor size was measured according to the RECIST 1.1 standards (14): the longest diameter of the tumor (Table 1). All children were divided into two groups according to the different location of the tumors: the MRTK group and the EERT group. The gender, age, clinical manifestations, tumor size, metastatic lesions, treatments, and overall survival cases were compared between the two groups. Once a child died, the follow-up will be terminated. Otherwise, the follow-up will be continued until July 31, 2021.
Table 1
| Case | Gender | Age of onset | Clinical manifestations | Location and size of tumor | Metastatic lesions | Immunohistochemistry | Treatments | Survival time | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 years | A visible or palpable mass, tenderness | Right occipital subcutaneous (5 cm × 5 cm) | None | INI-1 (−) | Enlarged excision; R or/and C | Alive (under chemotherapy, survived for 15 months) | Progress remission & no evidence of tumor recurrence |
| 2 | M | 10 months 27 days | Airway compression | Right mediastinum (10 cm × 6 cm) | None | INI-1 (−) | Biopsy | Dead (survived for 1 month 14 days) | Poor prognosis |
| 3 | M | 1 month 11 days | A visible or palpable mass | Lateral right orbit (1.9 cm × 1.9 cm × 3 cm) | None | SMARCB1 (−)/INI-1 (−) | Total excision; R or/and C | Alive (chemotherapy had been stopped, survived for 8 months with tumor) | Poor prognosis (tumor recurrence, after resection again, lymph node metastasis behind the right ear, suspicious metastases in the lung, bilateral clavicle, bilateral groin, swelling of the right face, and tumor spread throughout the body) |
| 4 | F | 3 years 1 month | Signs of spinal cord compression (ipsilateral limb hemiplegia) | Right neck (8 cm × 4 cm) | None | SMARCB1 (−)/INI-1 (−) | Total excision; R or/and C | Alive (under chemotherapy, survived for 7 months) | Progress remission & no evidence of tumor recurrence |
| 5 | F | 6 days | A visible or palpable mass | Right back of neck (5 cm × 6 cm) | None | SMARCB1 (−)/INI-1 (−) | Biopsy | Dead (survived for 1 month) | Poor prognosis |
| 6 | M | 8 months 22 days | Airway compression | Anterior mediastinum (8 cm × 4.5 cm) | None | – | Partial excision; chemotherapy | Dead (survived for 2 months) | Poor prognosis |
| 7 | F | 9 days | A visible or palpable mass | Multiple skin and subcutaneous parts of the body, Outer right thigh (4 cm × 5 cm) | None | INI-1 (−) | Partial excision | Dead (survived for 0.5 month) | Poor prognosis (multiple skin and subcutaneous parts metastases of the body) |
| 8 | F | 2 years 11 months | A visible or palpable mass, spinal cord compression (dysuria) | Pelvis (6 cm × 4 cm × 5.4 cm) | Multiple pelvic and lung metastases | INI-1 (−) | Biopsy; chemotherapy | Dead (survived for 2 months) | Poor prognosis (multiple pelvic and lung metastases) |
| 9 | M | 4 years 7 months | A visible or palpable mass, abdominal discomfort | Pelvis (7.7 cm × 6.2 cm × 6.3 cm) | None | INI-1 (−) | Total excision; R or/and C | Alive (chemotherapy was complete, survived for 22 months) | Completely remission & no evidence of tumor recurrence |
| 10 | M | 4 years 4 months | A visible or palpable mass, abdominal discomfort | Right lobe of liver (9.9 cm × 7.3 cm × 10.6 cm) | None | SMARCB1 (−)/INI-1 (−) | Total excision; R or/and C | Alive (under chemotherapy, survived for 19 months) | Progress remission & no evidence of tumor recurrence |
| 11 | M | 8 months 12 days | Vomit, hypercalcemia | Left kidney (6.7 cm × 2.5 cm × 2.7 cm) | Lung metastases (SIOP stage IV) | INI-1 (−) | Total excision; chemotherapy | Dead (survived for 4 months) | Poor prognosis (no significant regression of lung metastases) |
| 12 | M | 5 years | A visible or palpable mass, abdominal discomfort | Left kidney (11 cm × 11 cm × 17 cm) preoperative size after chemotherapy (12.2 cm × 8.3 cm × 9.5 cm) | Vein of right kidney, inferior vena cava, lung, bilateral lower limb bones metastases (SIOP stage IV) | INI-1 (−) | Totally excision; tumor thrombus removal; chemotherapy | Dead (survived for 6 months) | Poor prognosis (lung metastases increased) |
| 13 | F | 9 months 6 days | Diarrhea | Bilateral kidney: right (8.3 cm × 8 cm × 7.8 cm), preoperative size after chemotherapy: right (2 cm × 3.3 cm× 4.8 cm), left (0.6 cm × 0.9 cm) | Subcutaneous of the left back metastases (SIOP stage V) | INI-1 (−) | Preserving nephrectomy; R or/and C | Dead (survived for 12 months) | Poor prognosis (subcutaneous of the left back metastases, 6 months after Preserving nephrectomy, and lung metastases, 9 months after Preserving nephrectomy) |
| 14 | M | 1 year 8 months | Gross hematuria, fever, perirenal abscess | Left kidney (4.6 cm × 3.6 cm × 4.1 cm) | None (SIOP stage III) | INI-1 (−) | Total excision; chemotherapy | Dead (survived for 7 months) | Poor prognosis (lung metastases, 5 months after resection of left kidney) |
| 15 | F | 2 years | Gross hematuria | Right kidney (5.9 cm × 4.9 cm × 5.2 cm) | Inferior vena cava tumor thrombus (SIOP stage III) | SMARCB1 (−)/INI-1 (−) | Total excision; R or/and C | Alive (under chemotherapy, survived for 8 months) | Progress remission & no evidence of tumor recurrence |
| 16 | F | 7 months 20 days | A visible or palpable mass, decreased appetite | Right kidney (7.8 cm × 7.2 cm × 10.5 cm); preoperative size after chemotherapy (11.6 cm × 7 cm × 8 cm) | Lung metastases and hip suspicious metastases (SIOP stage IV) | SMARCB1 (−)/INI-1 (−) | Total excision; R or/and C | Dead (survived for 4 months) | Poor prognosis (lung metastases and hip suspicious metastases remain) |
MRT, malignant rhabdoid tumor; SIOP, International Society of Pediatric Oncology; R or/and C, radiotherapy or/and chemotherapy; M, male; F, female.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Children’s Hospital of Fudan University [No. (2021)360] and individual informed consent for this retrospective analysis was waived.
Efficacy evaluation and statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA) was used. The age, gender, tumor size, metastatic lesions and radiotherapy or/and chemotherapy of all children were included to construct a binary logistic regression analysis respectively to analyze the correlations between these and the survival of the children. The clinical characteristics of the groups were compared using ANOVA, Pearson’s chi-square test, continuity correction, or Fisher’s exact test. The Kaplan-Meier curve was drawn by Graphpad Prism 5.0 software, and P values <0.05 were considered to be statistically significant. All measurement data in this study are expressed as mean ± standard deviation (mean ± SD).
Results
Correlation analysis on clinical characteristics and survival
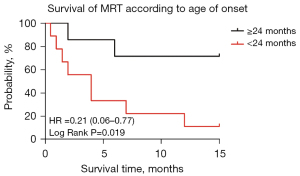
A total of 16 patients were followed up for at least 15 months. Among them, 9 were male and 7 were female with ages ranging from 6 days to 8 years, with an average age of 26.6±20.1 months. The age, gender, tumor size, metastatic lesions and radiotherapy or/and chemotherapy of all children were included to construct a binary logistic regression analysis respectively. Only the correlation between age and survival was confirmed [odds ratio (OR) =1.081, 95% CI: 1.004–1.164, P<0.05): 7 children aged ≥24 months, 9 children aged <24 months. The 1-year overall survival (OS) (the time since diagnosed) of 71.4% at the age of ≥24 months was significantly higher than the 1-year OS (11.1%) within age 24 months [hazard ratio (HR) =0.21 (0.06–0.77), log-rank P=0.019<0.05] (Figure 1).
Comparison of clinical symptoms, treatment, and survival cases of each group
There were 10 children in the EERT group, including 6 male (6/10) and 4 female (4/10). The average age of onset was 29.64±31.53 months, and the median was 22.95 months. The average tumor size in the early period of the disease was 6.93±2.37 cm. Clinical manifestations were 80% (8/10) of body surface masses, 30% (3/10) of pain, and compression symptoms (such as airway compression caused by mediastinal tumor; pain, hemiplegia and other neurological symptoms caused by tumor invasion of the spinal canal, hydronephrosis caused by mass compressing the ureter) was 20% (2/10), and neurological dysfunction was 20% (2/10). The masses mainly occurred in the neck, orbital, mediastinum and pelvic, and the incidence of bone metastasis was 10% (1/10), the systemic soft tissue was 10% (1/10), and the lung metastasis was 10% (1/10), while the incidence of no metastasis was 80% (8/10). In surgical treatment, biopsy accounts for 30% (3/10), partial excision 20% (2/10), and total excision 50% (5/10). There were 70% (7/10) with radiotherapy and chemotherapy, and 30% (3/10) without radiotherapy and chemotherapy. Eventually, 5 children survived and 5 died in this group, and the cases with deceased children had an average survival time of 1.40±0.65 months (Table 2).
Table 2
| Characteristics | EERT (n=10) | MRTK (n=6) |
|---|---|---|
| Gender | ||
| Male | 6 (60%) | 3 (50%) |
| Female | 4 (40%) | 3 (50%) |
| Average age of onset, months | 29.64±31.53 | 21.53±20.03 |
| Median age of onset, months | 22.95 | 14.6 |
| Clinical manifestations | ||
| Mass | 8 (80%) | 2 (33.3%) |
| Tenderness | 3 (30%) | 1 (16.7%) |
| Compression | 2 (20%) | 0 (0%) |
| Neurological dysfunction | 2 (20%) | – |
| Abdominal discomfort (vomiting, anorexia, etc.) | – | 3 (50%) |
| Gross hematuria | – | 2 (33.3%) |
| Fever, perirenal abscess | – | 1 (16.7%) |
| Hypercalcemia | – | 1 (16.7%) |
| Average tumor size from the beginning, cm | 6.93±2.37 | 8.83±4.49 |
| Metastatic lesions | ||
| Bone | 1 (10%) | 1 (16.7%) |
| Soft tissue | 1 (10%) | 1 (16.7%) |
| Lung | 1 (10%)* | 5 (83.3%) |
| None | 8 (80%)* | 1 (16.7%) |
| Surgical treatments | ||
| Biopsy | 3 (30%) | 0 (0%) |
| Partial excision | 2 (20%) | 1 (16.7%) |
| Total excision | 5 (50%) | 5 (83.3%) |
| Radiotherapy or/and chemotherapy | ||
| Yes | 7 (70%) | 6 (100%) |
| No | 3 (30%) | 0 (0%) |
| Overall survival | ||
| Alive | 5 (50%) | 1 (16.7%) |
| Dead | 5 (50%) | 5 (83.3%) |
| Average survival time, months | 1.40±0.65 * | 6.60±3.28 |
Compared with the MRTK group, *P<0.05. M, male; F, female; MRTK, malignant rhabdoid tumor of the kidney; EERT, extrarenal extracranial malignant rhabdoid tumor.
There were 6 children in the MRTK group, including 3 male (3/6) and 3 female (3/6). The average age of onset was 21.53±20.03 months, and the median age was 14.6 months. The average tumor size in the early period of the disease was 8.83±4.49 cm. The incidence of body surface masses was 33.3% (2/6), pain was 16.7% (1/6), and compression symptoms was 0% (0/0), gastrointestinal symptoms (vomiting, anorexia, etc.) was 50% (3/6), gross hematuria was 33.3% (2/6), perirenal abscess and fever was 16.7% (1/6), hypercalcemia was 16.7% (1/6). The incidence of bone metastasis was 16.7% (1/6), systemic soft tissue was 16.7% (1/6), lung metastasis was 83.3% (5/6), while the incidence of no metastasis was 16.7% (1/6). According to the International Society of Pediatric Oncology (SIOP) preoperative renal tumor staging standards, 2 patients were at stage III (33.3%), 3 patients were at stage IV (50%), 1 patient was at stage V (16.7%). In surgical treatment, biopsy accounts for 0% (0/6), partial excision 16.7% (1/6), and total excision 83.3% (5/6). All children received radiotherapy and chemotherapy (6/6) (100%). Finally, 1 child survived and 5 died in this group, and the cases with deceased children had an average survival time of 6.60±3.28 months (Table 2).
There was no significant difference in average age of onset between the EERT group and the MRTK group (P=0.712>0.05). The distant metastasis rate of tumor in the MRTK group (83.3%) was significantly higher than that in the EERT group (20%) (P=0.017<0.05). And the proportion of children with lung metastasis in the MRTK group was as much as 83.3%, which was significantly higher than that in the EERT group (10%) (P<0.05), but the related metastases and primary tumors did not disappear significantly after radiotherapy and chemotherapy (100%). The complete resection rate in the MRTK group was of 83.3% as compared to a higher complete resection rate of 50% in the EERT group, but the difference was not statistically significant. The average survival time of cases with deceased children in the MRTK group and EERT group were 6.60±3.28 and 1.40±0.65 months respectively, and the difference was statistically significant (P=0.008<0.05). There was no statistical difference between the two groups in regard to gender, tumor size, surgical treatments, and the cases of radiotherapy and chemotherapy (P>0.05) (Table 2).
In the MRTK group, 5 cases were completely excised (2 children aged ≥24 months, 3 children aged <24 months), all received radiotherapy and chemotherapy, but only 1 survived; while 5 cases received radiotherapy and chemotherapy in the EERT group that were completely excised and all alive, the survival cases were significantly more than in the MRTK group (P<0.05), and the EERT group has more cases (80%) than in the MRTK group (40%) at exceed 24 months old age, but there was no statistical difference (P>0.05) (Table 3).
Table 3
| Characteristics | EERT (n=5) | MRTK (n=5) |
|---|---|---|
| Age of onset | ||
| <24 months | 1 (20%) | 3 (60%) |
| ≥24 months | 4 (80%) | 2 (40%) |
| Survival cases with tumor total excision | ||
| Alive | 5 (100%)* | 1 (20%) |
| Dead | 0 (0%) | 4 (80%) |
| Radiotherapy or/and chemotherapy | ||
| Yes | 5 (100%) | 5 (100%) |
| No | 0 (0%) | 0 (0%) |
Compared with the MRTK group, *P<0.05. MRTK, malignant rhabdoid tumor of the kidney; EERT, extrarenal extracranial malignant rhabdoid tumor.
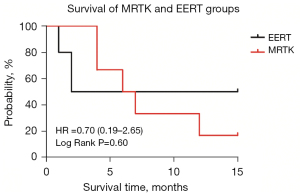
Children in EERT group had a 1-year overall survival of 50% as compared to Children in MRTK group with a 1-year overall survival of 16.7% [HR =0.70 (0.19–2.65), log-rank P=0.60>0.05] (Figure 2).
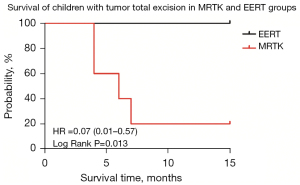
Children with tumor total excision in EERT group had a 1-year overall survival of 100% as compared to Children with tumor total excision in MRTK group with a 1-year overall survival of 20% [HR =0.07 (0.01–0.57), log-rank P=0.013<0.05] (Figure 3).
Discussion
MRT is a highly aggressive pediatric neoplasm that may arise from and/or share features with embryonic stem cells or germ cells (15), because of its very similar cell morphology to differentiating into rhabdoid cells (12), so it was named MRT. Studies have shown that the majority of MRT cases have a bi-allelic deletion of the SMARCB1/INI1 gene on chromosome 22q, suggesting a common biology underlying MRT (16), tumor immunohistochemistry of cases in our study were all negative in INI1 protein. However, some studies have shown that epithelioid sarcoma, renal medullary carcinoma may loss of INI1 expression (17-20). The diagnosis of MRT still requires tumor tissue microscopic morphology combined with immunohistochemical results (12).
Some studies believed that the clinical stage and age of MRTK are the main relevant factors that determine the prognosis: Patients younger than 6 months of age had a 2-year event-free survival (EFS) of 15% as compared to patients older than 27 months with a 2-year EFS of 48% (7). Our results are also similar to those reported in the literature, the correlation between age and survival was confirmed (OR =1.081, 95% CI: 1.004–1.164, P<0.05). The 1-year overall survival (OS) of 71.4% at the age of ≥24 months was significantly higher than the 1-year OS (11.1%) within 24 months of age [HR =0.21 (0.06–0.77), log-rank P=0.019<0.05] (Figure 1), it shows that the survival of children with MRT is closely correlated to the age factor, and the younger a patient at the time of onset is, the worse the prognosis will be. In our study, the ratio of male to female in children with MRTK was 1:1, and the median age of onset was 14.6 months, which is similar to the 13 months reported in literature (4). The ratio of male to female in children with EERT is 1.5:1, and the median age of onset is 22.95 months. The median age of onset of children with EERT reported in the literature is a huge span of time from 9 months to 7.5 years old (21,22), but usually later than MRTK (23), which is consistent with the results of our study. There was no significant difference in average age of onset between the EERT group (29.64±31.53 months) and the MRTK group (21.53±20.03 months) (P=0.712>0.05). The clinical manifestations of MRTK are mainly body surface masses (33.3%), pain (16.7%), compression symptoms (0%), gastrointestinal symptoms (vomiting, anorexia, etc.) (50%), gross hematuria (33.3%), perirenal abscess and fever (16.7%), hypercalcemia (16.7%), which is consistent with the typical clinical manifestations of MRTK as fever, hematuria, hypercalcemia, described by Amar et al. (24), but due to its untypicality, the first and early diagnosis of MRTK is usually the Wilms tumor. While the clinical manifestations of children with EERT mainly focus on body surface masses (80%), pain (30%), and compression symptoms (20%), which are usually indistinguishable from soft tissue sarcoma. The incidence of EERT has been reported to be 0.87% (26 out of 2,986 childhood sarcomas) (22), according to series studied in a large institute, and tumor location are usually neck, shoulders, trunk, para-spine, limbs, skin, genitourinary tract, liver and gastrointestinal, etc. (25). It is consistent with the tumor location of children with EERT in our study: neck, orbit, mediastinum and pelvic. Although there is no statistical difference between the average tumor size of MRTK (8.83±4.49 cm) and EERT (6.93±2.37 cm), it can be inferred that EERT may often occur on the body surface where mass is easier to find, which result in a smaller size of tumor. According to one of National Wilms’ Tumor Study (NWTS) Group trials, a total of 41 out of the 142 patients were stage IV, and among the stage IV patients, metastatic sites included lung (34 patients of 41, 82.9%), liver (three patients), bone (two patients), and brain (two patients) (8). In our study, 2 patients were at stage III (33.3%), 3 patients were at stage IV (50%), 1 patient was at stage V (16.7%) (SIOP preoperative renal tumor staging standards), the distant metastasis rate of children in the MRTK group (83.3%) is significantly higher than EERT group (20%) (P=0.017<0.05), and the proportion of children with lung metastasis in the MRTK group was as much as 83.3%, which was significantly higher than that in the EERT group (10%) (P<0.05), and was similar to the NWTS trials (82.9%). As MRTK is mainly adjacent to the renal vein and inferior vena cava, we infer that MRTK is more prone to blood metastasis, such as lung metastasis. EERT usually has a tendency to enlarged excision, because of highly malignant and invasive growth in the surrounding area, but it will relapse or metastasize soon after resection. It may be mainly caused by temporary suppression of immune function after operation, and release of various growth factors during wound healing.
There were no significant differences in the cases of biopsy, partial excision, and total excision between the MRTK group and the EERT group (P>0.05). The rate of total excision in the MRTK group (83.3%) was higher than that in the EERT group (50%), although the difference was not statistically significant (P>0.05), the kidney is usually removed together with the tumor in MRTK, it can ensure complete resection of the tumor, while EERT usually grows invasively and has unclear boundaries, so R0 resection cannot be guaranteed. In the MRTK group, 5 cases were completely excised, but only 1 survived; while 5 cases in the EERT group that were completely excised and all alive, the survival cases were significantly more than in the MRTK group (P<0.05), and the EERT group has more cases (80%) than in the MRTK group (40%) at exceed 24 months old age, but there was no statistical difference. It means that children with EERT may have a better prognosis if the tumor can be completely resected, while it may be susceptible to age factors because of a little more cases of children aged ≥24 months. Patients with MRTK were initially administered chemotherapy regimens as Wilms tumor which included combinations of the following drugs: carboplatin, etoposide, ifosfamide, doxorubicin, cyclophosphamide, actinomycin D, cisplatin, and vincristine (4), but poor prognosis. The chemotherapy described in the reports forms the basis for the current Children’s Oncology Group (COG) study of high-risk kidney tumors, which includes extracranial rhabdoid tumors, and the European Pediatric Soft Tissue Sarcoma Group protocol for extracranial MRTs: courses of vincristine, doxorubicin, and cyclophosphamide chemotherapy were alternated with courses of ifosfamide and etoposide in an intensive 2-weekly schedule with intermediate versus lower radiation doses (23). Recent clinical studies have shown that VDC regimen (vincristine, doxorubicin, cyclophosphamide) and ICE regimen (ifosfamide, carboplatin, etoposide) alternate chemotherapy or high-dose CEM regimen (carboplatin, Etoposide, melphalan) chemotherapy followed by autologous bone marrow transplantation (ABMT) can achieve good outcomes in the treatment of MRTK (4,26). Among the 10 children in the EERT group, biopsy is usually selected if the lesion is multi-site, and one-stage resection is selected for a single site. If its pathology is clear, but cannot be completely resected, it is partially resected and given further radiotherapy and chemotherapy. The management of EERT has shown the most disparity, as they have been variously viewed as undifferentiated sarcomas or carcinomas. Intergroup Rhabdomyosarcoma Study protocols within North America have evolved into COG trials, whereas in Europe, Malignant Mesenchymal Tumor (MMT) trials have latterly been superseded by the European Soft Tissue Sarcoma Study Group (EpSSG) guidelines. Although surgical timing may vary, there has been a common approach for chemotherapeutic agents, with combinations of vincristine, actinomycin D, doxorubicin, carboplatin, cyclophosphamide, and etoposide (27). In some pieces of literature, MRTK and EERT are usually both referred as extracranial MRTs and used the same regimens (4,24). However, Bourdeaut and colleagues reported 26 patients with EERT but only one patient remained free of disease after chemotherapies which focused on anthracyclines and ifosfamide-carboplatin-etoposide sequence (28), which means that the prognosis of EERT was still quite poor. For children with EERT, radiotherapy had no obvious effect on tumor regression and reduction of metastasis, while the effect of radiotherapy may be susceptible to age factors (3). In our study, we use the strategy of COG to treat with these cases above, such as carboplatin, etoposide, ifosfamide, cyclophosphamide, vincristine, and further radiotherapy, but the metastatic tumor and primary tumor of MRTK and EERT are both insensitive to radiotherapy and chemotherapy.
Children in EERT group had a 1-year overall survival of 50% as compared to Children in MRTK group with a 1-year overall survival of 16.7% [HR =0.70 (0.19–2.65), log-rank P=0.60>0.05] (Figure 2), but the average survival time of dead cases in MRTK group and EERT group were 6.60±3.28 and 1.40±0.65 months respectively, and the difference was statistically significant (P=0.008<0.05). Although metastasis is more common in the MRTK group, R0 resection is easier to achieve, while supplemented by preoperative neoadjuvant chemotherapy, which results in the longer survival period. However, there is still controversy about whether MRTK should be treated with preoperative neoadjuvant chemotherapy. Some scholars believed that removing the tumor completely was the most critical: Although preoperative chemotherapy reduced the tumor size and made it easier to excise, it also delayed the time of surgery and further reduced the ultimate survival of children with MRT (8,29). In our study, Children with tumor total excision in EERT group had a 1-year overall survival of 100% as compared to Children with tumor total excision in MRTK group with a 1-year overall survival of 20% [HR =0.07 (0.01–0.57), log-rank P=0.013<0.05] (Figure 3). However, there are several limitations to the results because rhabdoid tumor is very rare and the diagnosis in those clinical cases are sometimes difficult, which results in small sample size and the short follow-up.
In conclusion, MRTK and EERT are both rare and highly malignant, age of onset as a prognostic factor, children with MRT under 2 years of age have significantly worse prognosis than children exceeding 2 years of age. Although MRTK is more prone to lung metastasis than EERT, the survival time of MRTK is significantly longer than that of EERT children, which may be related to the R0 resection of the primary tumor, and the metastatic tumor and primary tumor of MRTK and EERT are both insensitive to radiotherapy and chemotherapy. Children with EERT may have a better prognosis if the tumor can be completely resected, while it may be susceptible to age.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2548/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2548/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2548/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2548/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Children’s Hospital of Fudan University [No. (2021)360] and individual informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Greeneway GP, Page PS, Patel V, et al. Atypical Teratoid/Rhabdoid Tumor of the Cerebellum in an Adult: Case Report and Literature Review. World Neurosurg 2021;145:57-63. [Crossref] [PubMed]
- Takahashi K, Nishihara H, Katoh M, et al. Case of atypical teratoid/rhabdoid tumor in an adult, with long survival. Brain Tumor Pathol 2011;28:71-6. [Crossref] [PubMed]
- Sultan I, Qaddoumi I, Rodríguez-Galindo C, et al. Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer 2010;54:35-40. [Crossref] [PubMed]
- Venkatramani R, Shoureshi P, Malvar J, et al. High dose alkylator therapy for extracranial malignant rhabdoid tumors in children. Pediatr Blood Cancer 2014;61:1357-61. [Crossref] [PubMed]
- Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer 1978;41:1937-48. [Crossref] [PubMed]
- Haas JE, Palmer NF, Weinberg AG, et al. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol 1981;12:646-57. [Crossref] [PubMed]
- van den Heuvel-Eibrink MM, van Tinteren H, Rehorst H, et al. Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr Blood Cancer 2011;56:733-7. [Crossref] [PubMed]
- Tomlinson GE, Breslow NE, Dome J, et al. Rhabdoid tumor of the kidney in the National Wilms' Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol 2005;23:7641-5. [Crossref] [PubMed]
- Reinhard H, Reinert J, Beier R, et al. Rhabdoid tumors in children: prognostic factors in 70 patients diagnosed in Germany. Oncol Rep 2008;19:819-23. [Crossref] [PubMed]
- Palmer NF, Sutow W. Clinical aspects of the rhabdoid tumor of the kidney: a report of the National Wilms' Tumor Study Group. Med Pediatr Oncol 1983;11:242-5. [Crossref] [PubMed]
- Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002;61:215-25; discussion 226-9. [Crossref] [PubMed]
- Wang H, Ma Y, Li J, et al. Extrarenal malignant rhabdoid tumor of childhood: a clinicopathologic analysis of 8 cases. Zhonghua Bing Li Xue Za Zhi 2014;43:805-808. [PubMed]
- Sato Y, Iizuka J, Imai K, et al. Case report of rhabdoid tumor of the kidney occurring in own kidney following kidney transplantation from the living relative. Nihon Hinyokika Gakkai Zasshi 2010;101:683-8. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Deisch J, Raisanen J, Rakheja D. Immunohistochemical expression of embryonic stem cell markers in malignant rhabdoid tumors. Pediatr Dev Pathol 2011;14:353-9. [Crossref] [PubMed]
- Roberts CW, Biegel JA. The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther 2009;8:412-6. [Crossref] [PubMed]
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 2009;33:542-50. [Crossref] [PubMed]
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005;65:4012-9. [Crossref] [PubMed]
- Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 1999;59:74-9. [PubMed]
- Sigauke E, Rakheja D, Maddox DL, et al. Absence of expression of SMARCB1/INI1 in malignant rhabdoid tumors of the central nervous system, kidneys and soft tissue: an immunohistochemical study with implications for diagnosis. Mod Pathol 2006;19:717-25. [Crossref] [PubMed]
- Kodet R, Newton WA Jr, Sachs N, et al. Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 1991;22:674-84. [Crossref] [PubMed]
- Oda Y, Tsuneyoshi M. Extrarenal rhabdoid tumors of soft tissue: clinicopathological and molecular genetic review and distinction from other soft-tissue sarcomas with rhabdoid features. Pathol Int 2006;56:287-95. [Crossref] [PubMed]
- Brennan B, Stiller C, Bourdeaut F. Extracranial rhabdoid tumours: what we have learned so far and future directions. Lancet Oncol 2013;14:e329-36. [Crossref] [PubMed]
- Amar AM, Tomlinson G, Green DM, et al. Clinical presentation of rhabdoid tumors of the kidney. J Pediatr Hematol Oncol 2001;23:105-8. [Crossref] [PubMed]
- Machado I, Noguera R, Santonja N, et al. Immunohistochemical study as a tool in differential diagnosis of pediatric malignant rhabdoid tumor. Appl Immunohistochem Mol Morphol 2010;18:150-8. [Crossref] [PubMed]
- Kerl K, Holsten T, Frühwald MC. Rhabdoid tumors: clinical approaches and molecular targets for innovative therapy. Pediatr Hematol Oncol 2013;30:587-604. [Crossref] [PubMed]
- Uwineza A, Gill H, Buckley P, et al. Rhabdoid tumor: the Irish experience 1986-2013. Cancer Genet 2014;207:398-402. [Crossref] [PubMed]
- Bourdeaut F, Fréneaux P, Thuille B, et al. Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer 2008;51:363-8. [Crossref] [PubMed]
- Brennan BM, Foot AB, Stiller C, et al. Where to next with extracranial rhabdoid tumours in children. Eur J Cancer 2004;40:624-6. [Crossref] [PubMed]


