
Dumbbell-type tenosynovial giant cell tumor of the buttocks: a report of two cases
Introduction
Tenosynovial giant cell tumor (TSGCT) is a benign tumor with localized aggressiveness which is classified into localized and diffuse types (1). Both are further divided into extra-articular and intra-articular types according to their growth sites. All of these entities are currently being classified as fibrous histiocytomas by the World Health Organization (2). TSGCT frequently affects young and middle-aged adults aged 20–50 years, with a similar or slightly more prevalence in females than the males (3). The annual incidence of TSGCT is 1.8 per million, with a recurrence rate of 9–46% (4). 85% of localized TSGCT occurs in the tendon sheath around the hand, foot, and wrist, while the diffuse type is mainly located in the large joints such as knee, hip, ankle, shoulder, and elbow, and rarely appeared complete extra-articular form, accounting for only about 5–15% (5). In rare cases, TSGCT can be completely located in the muscle or subcutaneous, usually in the lower extremities (6). In this study, we report two cases of complete extra-articular TSGCT at rare locations; both lesions were located in the deep layer of gluteal muscle, around the obturator or greater sciatic foramen, with a dumbbell-shaped anterior-posterior extension and no association with the hip joint. We described and summarized the imaging features, histopathological presentations, and prognosis of the disease, hoping to provide clinicians with experience and reference to reduce misdiagnosis and missed diagnosis. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2461/rc).
Case presentation
Case 1
A 23-year-old male patient was admitted to Tongji Hospital on May 30, 2014. He started experiencing pain and swelling in his left buttock 1 year ago, and a buttock mass was found 1 month ago. Physical examination revealed an irregular mass on the left buttock with tough, poor mobility and mild tenderness, no erythema in the skin of the buttock area, no ulceration or sinus tract, and the range of motion of left hip joint is normal. There was no trauma or disease history. Ultrasound examination revealed a hypoechoic range of about 9.8 cm × 3.7 cm at the root of the left thigh, with poorly defined boundaries with the surrounding muscle tissue, and anechoic areas and band-like segmentation were seen within the lesion. The CT angiography (CTA) examination showed an abnormal density mass in the left posterior inferior hip joint, external obturator muscle and femoral square muscle, which was co-supplied by the medial rotor femoral artery and the inferior gluteal artery branch of the internal iliac artery. Magnetic resonance imaging (MRI) examination showed that the left thigh root dumbbell-shaped mass was predominantly cystic solid tissue. on T1WI, it showed a mixed signal with predominantly isosignal and strip-like low signal separation in the lesion; on T2WI, it showed a distinct high signal with scattered low signal areas, with obvious boundary (Figure 1). MRI presentation needs to be differentiated with neurogenic or mesenchymal origin tumors. The patient’s blood tests, liver and kidney functions, coagulation tests and tumor marker test results were no significant abnormalities.
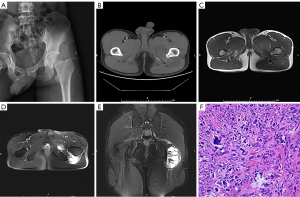
The patient was treated with left gluteal tumor resection + neurovascular exploration surgery on June 5, 2014. Intraoperatively, it was seen that the dumbbell-shaped tumor was located on the deep surface of gluteal muscle and penetrates to the front along the upper part of lesser trochanter, the upper boundary of the tumor was adjacent to the obturator foramen, the anterior adductor magnus completely wrapped the tumor, the posterior tumor capsule wrapped the sciatic nerve, and the tumor did not invade the hip joint. The posterior tumor was penetrated from above the lesser trochanter to the anterior, and the tumor was completely resected, and the sciatic nerve was completely dissociated and preserved. The resected tumor has a complete capsule, and after the tumor was dissected, yellow streak-like tissue with central necrosis can be seen. The histopathological examination showed that the tumor was greyish-white, solid, and tough, and necrosis was seen in the center of the lesion. They were mainly composed of monocytes, foam-like cells and scattered osteoclast-like multinucleated giant cells, with varying degrees of interstitial collagenization and lymphocytic infiltration. The pathological diagnosis was extra-articular pigmented villous nodular synovitis (diffuse-type TSGCT). The patient was followed up for approximately 7 years after surgery without tumor recurrence and the affected limb has good function.
Case 2
A 55-year-old male patient was admitted to our hospital on July 27, 2018. The patient complained of swelling and pain in the left buttock for half a year. Physical examination revealed a mass of about 5 cm × 5 cm in size on the left buttock with poor mobility and tenderness, no significant abnormalities in skin color and temperature of the buttock, and normal muscle strength and activity of the extremities. He had a history of abnormal glucose tolerance, hydronephrosis and tibial bone giant cell tumor. CTA examination revealed a soft tissue mass in the posterior and inferior regions of the left sacroiliac joint with irregular patterns and a branch of the left internal iliac artery penetrating the mass. MRI examination showed a soft tissue mass between the left hip joint and the gluteus muscle, involving the acetabulum, sacroiliac joint, and gluteus muscle. T1WI images show the lesions were mixed signals dominated by isometric signals, with clear boundaries and a capsule (Figure 2). MRI presentation needs to be differentiated from giant cell tumor of soft tissue or mesenchymal-derived tumor. Laboratory tests showed elevated AFP protein levels and other tests were normal.
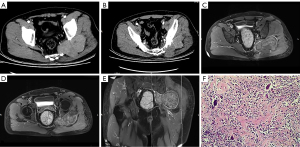
A biopsy of the left gluteus tumor was performed on August 1, 2018, and the biopsy pathology considered diffuse-type TSGCT. Subsequently, a left sciatic foramen magnum tumor resection + neurovascular exploration was performed on August 15, 2018. Intraoperatively, the tumor was seen to be located at the foramen magnum, yellowish-brown and dumbbell-shaped, with the sciatic nerve compressed by the mass at a deep level and the bone of the left sacroiliac joint was eroded and destructed, and the tumor was completely excised outside the envelope and the iliac and sacral lesions were scraped. The histopathological section showed gray-white and gray-yellow nodule-like tissue with a slightly hard or tough texture, which seems to have a capsule. The histological features were similar to case 1. Immunohistochemical results showed: CD68 (+), CD163 (+), MDM2 (−), CDK4 (−), p63 (−), p53 (−), DES (−), SATB2 (−), Ki67 (+), MCM2 (+). The pathological diagnosis was diffuse type TSGCT. The patient presented with tumor recurrence 1 year after surgery and underwent another surgery on October 17, 2019 for left greater sciatic foramen tumor resection + sciatic nerve exploration, and the surgical pathology was considered as a recurrence of diffuse-type TSGCT, and malignancy could not be completely excluded. Three weeks after surgery, the patient went to the oncology department of our hospital for further radiotherapy. The whole course of radiotherapy was 3000 cGY, 200 cGy each time, 5 times a week. And up to now, the patient has not recurred again.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images.
Discussion
TSGCT is rare in extra-articular and more rarely in purely intramuscular or subcutaneous. Most of them are located in the lower extremities, such as thighs, buttocks and legs, but it can occur in all muscles (5). Takeuchi et al. (1) reported two cases of complete extra-articular localized TSGCT, one located in the left C1 paravertebral plate soft tissue and unrelated to the synovial joint and the other located in the rectus femoris muscle. In addition, reports of complete extra-articular diffuse-type TSGCT located within the soft tissues of the thigh or subcutaneously have been seen (7,8). Osanai et al. (9) reported one case of diffuse-type TSGCT located within the right gluteus maximus muscle with a lesion located completely outside the joint and not associated with the joint capsule and synovium. This is similar to the region reported in this study, in which one case was located in the medial region of the left lesser trochanter and the deep surface of the gluteus, which was completely extra-articular and did not invade the hip joint, while the other case was located around the greater sciatic foramen in the deep surface of the left gluteus, with a dumbbell-shaped lesion expanding from posterior to anterior and invading the left sacroiliac joint, but no significant association with the joint capsule or bursa was seen. In addition, complete extra-articular TSGCT can also occur in soft tissues such as the pes anserinus tendon, subacromial bursa, temporalis muscle, external auditory canal, and paravertebral muscles (3,10-12). The current view is that extra-articular TSGCT may originate from the synovial tissue of the bursa and tendon sheath (5,13).
TSGCT was initially thought to be a non-neoplastic, inflammatory proliferative synovial lesion (14), and later revealed the presence of chromosomal abnormalities, aggressive growth, potential recurrence and metastasis, and is now mostly considered to be a benign neoplastic lesion (15). The etiology of TSGCT is unclear and there are several perspectives including: abnormal lipid metabolism; chronic trauma and recurrent bleeding irritation causing reactive hyperplasia; inflammatory reactions; and benign tumors of synovial, vascular, or fibrous histiocyte origin (16). Many studies have reported a history of trauma in patients with TSGCT (13,16), and some have even reported a history of trauma in more than 50% of patients (17). Nevertheless, it still cannot be concluded that trauma is the direct cause of the lesion. Both patients in this study had no definitive history of trauma to the affected area. Accumulating evidence suggests that TSGCT is caused by a t(1,2) chromosomal translocation, CSF1 gene fusion with COL6A3 gene promoter resulting in monocyte overexpression of CSF1, which in turn recruits and activates monocyte macrophages to express CSF1R, promotes their maturation and proliferation, and ultimately leads to tumorigenesis (7,18).
The correct diagnosis of TSGCT requires a comprehensive analysis of clinical manifestations, imaging features and pathological findings. Its clinical symptoms are non-specific and greatly vary depending on the location and course of the disease, mostly as a painless slow-growing mass. Doppler ultrasound can clarify the site, size and internal blood flow signal of the lesion and can be used as a basic screening tool and guide clinical puncture biopsy (19). X-ray and CT examinations are important in clinical practice as they can detect an increased density soft tissue mass and clarify the presence of calcification and the erosion and destruction of adjacent bone. It has been reported that in chronic cases, bone abnormalities can be detected on X-ray in 1/3 of patients (20). Magnetic resonance imaging can clarify the extent of the lesion, the composition and characteristics of the tissue contents, and the relationship with adjacent important structures, which is valuable for developing treatment plans and planning surgery. TSGCT generally shows low or moderate signal on T1WI, while T2WI manifestation is highly variable depending on the composition of hemosiderin, lipids, collagenous fibrous, blood, and cell amounts, and mostly shows mixed or heterogeneous signals (12). CTA is also an option to show the blood supply of the lesion and the relationship with the adjacent important vessels, to clarify the need for embolization, and to guide the intraoperative avoidance of important vascular, thus helping to minimize the risk of surgery. The two patients admitted to our hospital had CTA examination and embolization of major supply vessels before surgery because of the special location and extent of the tumor, and the lesions were finally resected successfully. Pathological examination is the gold standard for TSGCT diagnosis, and immunohistochemical examination can be further performed for cases with unclear microscopic histopathological features. It has been reported that the immunophenotype of TSGCT is mainly monocyte-macrophage-like and limited myofibroblastic differentiation features (21). In our study, one patient underwent immunohistochemical test and the result was positive for CD68, CD163, MCM2 and Ki-67, which was similar to the results of other studies (22,23). CD68 is a positive marker of osteoclast-like multinucleated giant cells and CD163 is a marker of small histiocytoid cells, both of which can be used in the differential diagnosis of TSGCT.
Due to the atypical clinical symptoms and imaging features, TSGCT can be easily confused with giant cell-rich tumors or mesenchymal soft tissue tumors. Especially for extra-articular TSGCT, it needs to be differentiated from giant cell tumor of soft tissue (GCTST), undifferentiated pleomorphic sarcoma (UPS)/malignant fibrous histiocytoma (MFH), rhabdomyosarcoma, leiomyosarcoma and synovial sarcoma. GCTST are most commonly seen in middle-aged and elderly patients, and often located in the subcutaneous of the limbs. Microscopically, it is mostly nodular, mainly composed of monocytes and multinucleated osteoclast like giant cells, which are evenly distributed among monocytes. In contrast, extra-articular TSGCT occurs in middle-aged and young adults. Microscopically, 2 different types of monocytes are seen, most of which are A-type synoviocyte-like monocytes and a few are large dendritic monocytes with lobulated or kidney-shaped nuclei, vesicular chromatin, containing distinct eosinophilic nuclei, occasional paranuclear eosinophilic inclusions, abundant cytoplasm, and peripheral ring composed of hemosiderin particles. MFH/UPS is a malignant tumor of mesenchymal origin. It is common in patients aged 50–70 years. It mainly occurs in the extremities and retroperitoneum, with a histological composition of multiple components. Under microscope, the cells are more atypical and mitotic. Rhabdomyosarcoma is more common in the extremities, and the lesions are usually large and lobulated, but rarely invades the adjacent bone. Leiomyosarcoma of the extremities is most common in middle-aged and elderly people. Microscopically, it consists of cigar-like spindle cells with bundles or woven arrangement, with obvious cell atypical and mitotic, and is mostly positive for smooth muscle actin (SMA) by immunohistochemistry. Synovial sarcoma is most commonly located around the knee joint. In 20–30% of cases, striped or speckled calcification can be seen in the lesion on x-ray or CT. Cystic necrosis, hemorrhage, and fluid-fluid levels can be seen on MRI in some cases, and the “triple signal sign” is a typical symbol. Microscopy shows biphasic differentiation of tumor cells, and immunohistochemistry shows biphasic positivity of mesenchymal and epithelial components. Extraskeletal osteosarcoma is a rare malignant mesenchymal tumor that occurs in middle-aged and elderly people, with characteristic cotton-like or patchy tumor bone visible on X-ray or CT. Histologically, it is mainly composed of spindle cells, cartilage, and osteoid matrix. In addition, extra-articular TSGCT around the hip need to be differentiated from metastatic tumors, malignant peripheral nerve sheath tumors, fibrosarcomas, and hemangiomas.
In conclusion, extra-articular TSGCT located purely in the muscle is rare and easily misdiagnosed. For soft tissue tumors located in the gluteus, without any obvious association with the hip joint and bursa, and showing low or equal signal on T1WI and mixed signal on T2WI, the diagnosis of extra-articular TSGCT needs to be considered. Accurate diagnosis needs to be integrated with medical history, clinical manifestations, imaging examinations and pathological findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2461/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2461/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takeuchi A, Yamamoto N, Hayashi K, et al. Tenosynovial giant cell tumors in unusual locations detected by positron emission tomography imaging confused with malignant tumors: report of two cases. BMC Musculoskelet Disord 2016;17:180. [Crossref] [PubMed]
- Staals EL, Ferrari S, Donati DM, et al. Diffuse-type tenosynovial giant cell tumour: Current treatment concepts and future perspectives. Eur J Cancer 2016;63:34-40. [Crossref] [PubMed]
- Madruga Dias J, Costa MM, Duarte A, et al. Localized Pigmented Villonodular Synovitis of the shoulder: a rare presentation of an uncommon pathology. Acta Med Port 2013;26:459-62. [PubMed]
- Yang X, Yao L, Yu T, et al. Case Report: Extra-Articular Diffuse Tenosynovial Giant Cell Tumor of the Temporomandibular Joint. Front Oncol 2021;11:643635. [Crossref] [PubMed]
- Somerhausen NS, Fletcher CD. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am J Surg Pathol 2000;24:479-92. [Crossref] [PubMed]
- Ushijima M, Hashimoto H, Tsuneyoshi M, et al. Giant cell tumor of the tendon sheath (nodular tenosynovitis). A study of 207 cases to compare the large joint group with the common digit group. Cancer 1986;57:875-84. [Crossref] [PubMed]
- Lopez L, Schoedel K, John I. Giant Cell Poor Extra-Articular Diffuse-Type Tenosynovial Giant Cell Tumor With Extensive Desmin Expression: A Potential Diagnostic Pitfall With Cytogenetic Confirmation. Int J Surg Pathol 2019;27:59-61. [Crossref] [PubMed]
- Sanghvi DA, Purandare NC, Jambhekar NA, et al. Diffuse-type giant cell tumor of the subcutaneous thigh. Skeletal Radiol 2007;36:327-30. [Crossref] [PubMed]
- Osanai T, Suzuki H, Hiraga H, et al. Extra-articular diffuse-type tenosynovial giant cell tumor with benign histological features resulting in fatal pulmonary metastases. J Orthop Surg (Hong Kong) 2017;25:2309499017690323. [Crossref] [PubMed]
- Shahriari S, Ederle A, Botros J, et al. Tenosynovial Giant Cell Tumor in an Infant. J Craniofac Surg 2020;31:1760-2. [Crossref] [PubMed]
- Maghari A, Zaleski TA, Jow T, et al. Tenosynovial Giant Cell Tumor in the Dermis of the External Auditory Meatus. Skinmed 2016;14:48-51. [PubMed]
- Kim YJ, Hong JH, Park JH, et al. Tenosynovial giant cell tumor of the upper cervical spine arising from the posterior atlanto-occipital membrane: a case report. Skeletal Radiol 2021;50:451-5. [Crossref] [PubMed]
- Hepp P, Engel T, Marquass B, et al. Infiltration of the pes anserinus complex by an extraarticular diffuse-type giant cell tumor (D-TGCT). Arch Orthop Trauma Surg 2008;128:155-8. [Crossref] [PubMed]
- Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented Villonodular Synovitis, Bursitis and Tenosynovitis. Arch Pathol 1941;31:731-65.
- Rubin BP. Tenosynovial giant cell tumor and pigmented villonodular synovitis: a proposal for unification of these clinically distinct but histologically and genetically identical lesions. Skeletal Radiol 2007;36:267-8. [Crossref] [PubMed]
- Gong ZC, Lin ZQ, Moming A, et al. Extra-articular diffuse tenosynovial giant cell tumour of the infratemporal fossa: report of a case and literature review. Int J Oral Maxillofac Surg 2010;39:820-4. [Crossref] [PubMed]
- Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine (Baltimore) 1980;59:223-38. [Crossref] [PubMed]
- West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci U S A 2006;103:690-5. [Crossref] [PubMed]
- Gouin F, Noailles T. Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis). Orthop Traumatol Surg Res 2017;103:S91-7. [Crossref] [PubMed]
- Ofluoglu O. Pigmented villonodular synovitis. Orthop Clin North Am 2006;37:23-33. [Crossref] [PubMed]
- Maluf HM, DeYoung BR, Swanson PE, et al. Fibroma and giant cell tumor of tendon sheath: a comparative histological and immunohistological study. Mod Pathol 1995;8:155-9. [PubMed]
- Nguyen TT, Schwartz EJ, West RB, et al. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol 2005;29:617-24. [Crossref] [PubMed]
- Kondo R, Akiba J, Hiraoka K, et al. Malignant diffuse-type tenosynovial giant cell tumor of the buttock. Pathol Int 2012;62:559-64. [Crossref] [PubMed]


