
Serum alpha-fetoprotein as a predictive biomarker for tissue alpha-fetoprotein status and prognosis in patients with hepatocellular carcinoma
Introduction
Liver cancers are estimated to rank the sixth among newly diagnosed cancers and the fourth cause of cancer-related death in the world with over 8.4 million new cases and 7.8 million deaths annually (1). Hepatocellular carcinoma (HCC) accounts for the most common type of primary liver cancers which is most caused by hepatitis B or C virus (HBV or HCV) infection or alcohol abuse, with poor prognosis due to the lack of specific symptoms at the early stage (2). Therefore, Asian-Pacific Association for the Study of the Liver (APASL) guidelines recommended using ultrasonography (US) and serum alpha-fetoprotein (AFP) tests for early diagnosis of individuals with the above liver diseases (3).
AFP is the most widely used biomarker for HCC, even though it has been reported that up to 20% of HCC patients’ tumor cells do not secrete AFP proteins (4). A previous study reported that a cutoff value of AFP of 90.0 ng/mL had a sensitivity of 0.609, and specificity of 0.818 for diagnosing HCC in 121 enrolled patients, and the receiver operating characteristic (ROC) curve of AFP for diagnosis of HCC was 0.746 (95% CI: 0.674–0.818) (5). Serum AFP level can indirectly reflect the ability of tumor cells to synthesize and process AFP protein. Combined with imaging techniques, variation tendency of serum AFP plays a critical role in surveillance of individuals with HCC risks and diagnosis of HCC. More importantly, AFP has been widely accepted as a prognostic predictor for HCC in recent researches.
A retrospective study reported that preoperative serum AFP level greater than 100 ng/mL was associated with a higher rate of recurrence of HCC after hepatectomy (6). According to the results of the above study, preoperative serum AFP level can help surgeon to make a comprehensive treatment schedule based on surgery. Moreover, tissue AFP staining status of specimens obtained by liver biopsy is useful in the histologic early diagnosis of HCC (7). The expression level of AFP protein in tumor tissue was significantly higher than that in normal tissue, but the expression level of AFP in carcinoma and para-carcinoma tissue showed no significant difference in the same patients (8). AFP protein expression rate in HCC is 25% to 50%, and the positive AFP status is negatively correlated with tumor histological grade (9,10). However, serum AFP level is negative in 20% HCC patients clinically, but those patients’ tissue AFP status may be positive or negative. Therefore, the clinical association among serum AFP level and tissue AFP status is not clear.
Here, we conducted a retrospective study of patients with HCC to investigate the relationship between preoperative serum AFP concentration and tissue AFP status and to identify the prognostic value of serum and tissue AFP. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2334/rc).
Methods
Patient cohort
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 19-010/1795), and written informed consents were obtained from all the patients during the hospital stay. Patients with primary HCC who had been treated with surgical resection by the same single-surgeon team at our institution between January 2012 and December 2018 were identified. Inclusion criteria were: (I) patients who underwent hepatectomy; (II) pathological diagnosis was HCC; (III) postoperative survival more than 1 month. Exclusion criteria were: (I) a history of prior cancer surgery; (II) combined with other local therapy during surgery; (III) extrahepatic metastases detected during surgery; (IV) had missing data that made it impossible to evaluated. Clinical data collected from eligible patients included the following: age, sex, HBV, HCV, tumor number, tumor size, Barcelona clinic liver cancer (BCLC) stage, histological grade, preoperative serum AFP level and postoperative tissue AFP status. Overall survival (OS) was defined as the time from the end of surgery to the death of any cause. The rules of follow-up were described in our previous report in detail (11) and the treatment after recurrence including surgery, radiofrequency ablation, transhepatic arterial chemotherapy and embolization (TACE), radiation and systemic therapy.
Serum and tissue AFP assay
Preoperative serum AFP concentrations were measured by electrochemiluminescence immunoassay (ECLI) with a normal range of 0–7 ng/mL (the reference range of ECLI kits) and AFP value could be collected from preoperative laboratory results. Postoperative tissue AFP status was measured by immunohistochemistry (IHC) that was performed using an indirect peroxidase method. The sections (5 µm) of cancer tissues were deparaffinized with xylene and rehydrated in citrate buffer. Next, the sections were incubated at 4 ℃ overnight with primary AFP antibodies (1:50 dilution; ZSGB-Bio, Beijing, China) raised in rabbit. After incubating with a secondary antibody, diaminobenzidine was used to develop the antigen‐antibody reactions. AFP protein is cytoplasmic staining and were scored 0–3 according to tumor specimens with AFP IHC staining intensity. The staining intensity of 2+ or more were judged as AFP-positive status, others were AFP-negative status. The IHC process was conducted at the Department of Pathology, Cancer Hospital, Chinese Academy of Medical Sciences. Tissue AFP expression was reviewed by experienced pathologists.
Statistical analysis
Continuous data are presented as median and interquartile range (IQR), and categorical data are presented as frequency and percentage. OS was analyzed using the Kaplan-Meier method and log-rank tests were used for comparisons between two groups. ROC curves were plotted to investigate the predictive value of serum AFP for tissue AFP status. Youden’s index (sensitivity + specificity −1) was used to determine the optimal cut-off value of serum AFP from the ROC plot. The correlations between serum or tissue AFP and clinicopathological variables were investigated using Chi-square test. Univariate Cox proportional hazards regression analysis was performed for various risk factors. Variables shown to be statistically different (P<0.1) through univariate Cox regression analysis were included in multivariate Cox regression analysis to identify the independent prognostic factors and estimate the hazard ratio (HR) and 95% confidence interval (CI) of covariates. P<0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS (version 22, Armonk, NY, USA).
Results
Patient characteristics
A total of 248 patients with HCC were enrolled in the study and 204 (82.3%) were male. The median age was 57.3 years (IQR: 49.5–63.9 years). One hundred seventy-eight (71.8%) patients were HBV positive and 16 (6.5%) patients were HCV positive. More than 90% patients had single tumor, and patients with tumor <5 cm accounted for 70.6%. One hundred seventy-four (70.2%) patients were with tissue AFP-negative status and 74 (29.8%) patients were tissue AFP positive. Eighty-one (32.7%) patients were with AFP <7 ng/mL and 167 (67.3%) were with AFP ≥7 ng/mL. The median serum AFP level was 15.9 ng/mL (IQR: 4.8–239.3 ng/mL). The details were showed in Table 1.
Table 1
| Variables | All (N=248) | Percent (%) |
|---|---|---|
| Age (years) | ||
| Median (IQR) | 57.3 (49.5–63.9) | – |
| <60 | 158 | 63.7 |
| ≥60 | 90 | 36.3 |
| Sex | ||
| Male | 204 | 82.3 |
| Female | 44 | 17.7 |
| HBV | ||
| Positive | 178 | 71.8 |
| Negative | 70 | 28.2 |
| HCV | ||
| Positive | 16 | 6.5 |
| Negative | 232 | 93.5 |
| Tumor number | ||
| Single | 232 | 93.5 |
| Multiple | 16 | 6.5 |
| Tumor size (cm) | ||
| <5 | 175 | 70.6 |
| ≥5 | 73 | 29.4 |
| BCLC stage | ||
| 0 | 35 | 14.1 |
| A | 138 | 55.6 |
| B | 75 | 30.2 |
| Histological grade | ||
| I | 32 | 12.9 |
| II | 143 | 57.7 |
| III | 73 | 29.4 |
| Tissue AFP status (IHC) | ||
| Negative | 174 | 70.2 |
| Positive | 74 | 29.8 |
| Serum AFP (ng/mL) | ||
| AFP <7 | 81 | 32.7 |
| AFP ≥7 | 167 | 67.3 |
| Serum AFP (ng/mL) | ||
| Median (IQR) | 15.9 (4.8–239.3) | – |
| Overall survival (months) | ||
| Median (IQR) | 42 (31–60) | – |
IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona clinic liver cancer; AFP, alpha-fetoprotein; IHC, immunohistochemistry.
Association between serum AFP level and tissue AFP status
The median serum AFP level in tissue AFP-positive and AFP-negative patients were 506.7 ng/mL (IQR: 181.7–3,296.3 ng/mL) and 7.7 ng/mL (IQR: 3.2–33.3 ng/mL), respectively. The serum AFP level in patients with tissue AFP-positive HCC was higher than those with tissue AFP-negative HCC (P<0.0001, Figure 1A). To identify the optimal cut-off value of serum AFP for tissue AFP status, the ROC plot showed an area under curve value of 0.92 (95% CI: 0.88–0.95, P<0.0001, Figure 1B). Youden’s index yielded an optimal cut-off value of 92.33 ng/mL, with a sensitivity and specificity of 0.84 (95% CI: 0.74–0.90) and 0.88 (95% CI: 0.82–0.92), respectively.
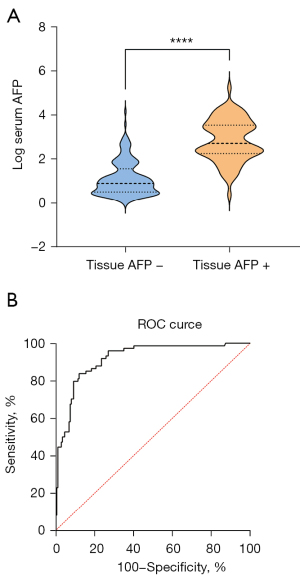
Correlations between serum or tissue AFP and clinicopathological variables
We divided the enrolled patients into high-and low serum AFP groups based on the cut-off value of 92.33 ng/mL. High serum AFP concentrations were significantly correlated with positive HBV (P=0.012), tumor size (P=0.025), histological grade (P=0.001). Similarly, patients were divided into positive and negative tissue AFP groups. Tissue AFP-positive status was associated with positive HBV (P=0.006), tumor number (P=0.033), histological grade (P<0.001, Table 2).
Table 2
| Variables | Serum AFP concentration (ng/mL) | P | Tissue AFP expression (IHC) | P | ||
|---|---|---|---|---|---|---|
| AFP <92.33, N=165 (66.5%) | AFP ≥92.33, N=83 (33.55%) | AFP negative, N=174 (70.2%) | AFP positive, N=74 (29.8%) | |||
| Age (years), n (%) | ||||||
| <60 | 99 (60.0) | 59 (71.1) | 0.087 | 105 (60.3) | 53 (71.6) | 0.091 |
| ≥60 | 66 (40.0) | 24 (28.9) | 69 (39.7) | 21 (28.4) | ||
| Sex, n (%) | ||||||
| Male | 141 (85.5) | 63 (75.9) | 0.063 | 147 (84.5) | 57 (77.0) | 0.160 |
| Female | 24 (14.5) | 20 (24.1) | 27 (15.5) | 17 (23.0) | ||
| HBV, n (%) | ||||||
| Positive | 110 (66.7) | 68 (81.9) | 0.012 | 116 (66.7) | 69 (93.2) | 0.006 |
| Negative | 55 (33.3) | 15 (18.1) | 58 (33.3) | 21 (6.8) | ||
| HCV, n (%) | ||||||
| Positive | 11 (6.7) | 5 (6.1) | 0.846 | 13 (7.5) | 3 (4.1) | 0.316 |
| Negative | 154 (93.3) | 78 (93.9) | 161 (92.5) | 71 (95.9) | ||
| Tumor number, n (%) | ||||||
| Single | 154 (93.3) | 78 (93.9) | 0.846 | 159 (91.4) | 73 (98.6) | 0.033 |
| Multiple | 11 (6.7) | 5 (6.1) | 15 (8.6) | 1 (1.4) | ||
| Tumor size (cm), n (%) | ||||||
| <5 | 124 (75.2) | 51 (61.4) | 0.025 | 122 (70.1) | 53 (71.6) | 0.812 |
| ≥5 | 41 (24.8) | 32 (38.6) | 52 (29.9) | 21 (28.4) | ||
| BCLC stage, n (%) | ||||||
| 0 | 26 (15.8) | 9 (10.8) | 0.062 | 23 (13.2) | 12 (16.2) | 0.700 |
| A | 97 (58.8) | 41 (49.4) | 96 (55.2) | 42 (56.8) | ||
| B | 42 (25.4) | 33 (39.8) | 55 (31.6) | 20 (27.0) | ||
| Histological grade, n (%) | ||||||
| I | 29 (17.6) | 3 (3.6) | 0.001 | 31 (17.8) | 1 (1.4) | <0.001 |
| II | 97 (58.8) | 46 (55.4) | 101 (58.0) | 42 (56.8) | ||
| III | 39 (23.6) | 34 (41.0) | 42 (24.2) | 31 (41.8) | ||
AFP, alpha-fetoprotein; IHC, immunohistochemistry; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona clinic liver cancer.
Prognostic factors of HCC patients
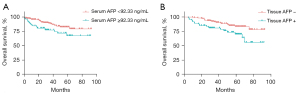
The Kaplan-Meier curve was used to compare survival of patients with two types of AFP measurement separately. According to Youden’s index in the ROC plot, the optimal cut-off value of serum AFP for tissue AFP status was 92.33 ng/mL, patients with serum AFP <92.33 ng/mL had significantly longer survival compared with patients with serum AFP ≥92.33 ng/mL (P=0.004, Figure 2A). Further, HCC patients with tissue AFP-negative had longer survival than tissue AFP-positive patients (P=0.016, Figure 2B).
After a median follow-up time of 42 months (IQR: 31–60 months), we tried to investigate the effect of clinicopathological variables including serum AFP level and tissue AFP status on survival. Univariate and multivariate Cox regression analysis demonstrated that HBV and tumor size were significantly correlated with OS. Positive HBV (Positive; HR 3.496; 95% CI: 1.349–9.064; P=0.010) and larger tumor size (≥5; HR 2.617; 95% CI: 1.372–4.992; P=0.003) were independent factors of OS. Age, sex, HCV, tumor number, BCLC stage, histological grade, tissue AFP status and serum AFP level did not affect OS independently (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | |||||||
| <60 | 1 | 0.484–1.749 | 0.800 | – | – | – | |
| ≥60 | 0.921 | – | |||||
| Sex | |||||||
| Male | 1 | 0.786–1.643 | 0.497 | – | – | – | |
| Female | 1.136 | – | |||||
| HBV | |||||||
| Negative | 1 | 1.281–8.299 | 0.013 | 1 | 1.349–9.064 | 0.010 | |
| Positive | 3.260 | 3.496 | |||||
| HCV | |||||||
| Negative | 1 | 0.139–2.394 | 0.449 | – | – | – | |
| Positive | 0.578 | – | |||||
| Tumor number | |||||||
| Single | 1 | 0.375–3.942 | 0.745 | – | – | – | |
| Multiple | 1.215 | – | |||||
| Tumor size (cm) | |||||||
| <5 | 1 | 1.188–4.006 | 0.012 | 1 | 1.372–4.992 | 0.003 | |
| ≥5 | 2.182 | 2.617 | |||||
| BCLC stage | |||||||
| 0 | 1 | – | – | – | – | – | |
| A | 1.840 | 0.550–6.157 | 0.323 | – | – | – | |
| B | 2.938 | 0.861–10.032 | 0.085 | – | – | – | |
| Histological grade | |||||||
| I | 1 | – | – | 1 | – | – | |
| II | 8.302 | 1.130–60.963 | 0.037 | 5.604 | 0.749–41.955 | 0.093 | |
| III | 6.030 | 0.777–46.790 | 0.086 | 3.165 | 0.397–25.223 | 0.277 | |
| Tissue AFP status (IHC) | |||||||
| Negative | 1 | 1.299–4.361 | 0.005 | 1 | 0.892–4.576 | 0.092 | |
| Positive | 2.380 | 2.020 | |||||
| Serum AFP (ng/mL) | |||||||
| <92.33 | 1 | 1.129–3.796 | 0.019 | 1 | 0.426–2.235 | 0.955 | |
| ≥92.33 | 2.070 | 0.976 | |||||
HCC, hepatobiliary carcinoma; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona clinic liver cancer; AFP, alpha-fetoprotein; IHC, immunohistochemistry.
Discussion
The current study was important because it specifically focused on serum and tissue AFP. This is the first study, to the best of our knowledge, to test the predictive value of serum AFP concentration for tissue AFP status of HCC. Previous study reported that AFP protein could be detected in tumor tissues in patients with high serum AFP levels, but not in adjacent normal liver tissues or tumor tissues in patients with low serum AFP levels (12). Our results demonstrated that AFP protein positive expression rate in is 29.8%, and patients with AFP-positive status had higher serum AFP concentration than patients with AFP-negative status, and the optimal cut-off value of serum AFP is 92.33 ng/mL for tissue AFP status, and the ROC plot proved to be a sensitivity and specificity of 0.84 and 0.88, respectively. There was a significant association between serum AFP levels and tissue AFP status.
Additionally, the present study concluded that HCC patients with serum AFP ≥92.33 ng/mL had poorer OS. Investigators have reported that serum AFP level was associated with HCC patients’ prognosis. Among 852 patients, patients with high AFP (>10 ng/mL) levels had worse 3-year post-recurrence survival compared with individuals with low AFP (<10 ng/mL) levels. After adjusting for competing risk factors, patients with AFP >10 ng/mL at the time of recurrence were independently associated with almost twofold higher hazards of death in the post-recurrence setting (13).
Apart from serum AFP, there are some biomarkers reported trustworthily in recent studies, but they are still associated with AFP. Park et al. (14) demonstrated that yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) were both expressed in 13.4% and 4.3% HCCs respectively. More importantly, YAP/TAZ-positive HCCs were significantly associated with higher serum AFP levels (P=0.024). Similarly, Li et al. (15) found that methyltransferase-like 18 (METTL18) is overexpressed in HCC and higher expression of METTL18 in HCC is positively related to AFP >400 ng/mL (P<0.05), and higher expression of METTL18 was significantly associated with poor prognosis of patients with HCC. This study showed that serum AFP concentration and tissue AFP status were correlated with clinicopathological variables including HBV, tumor number and size, and histological grade, but not with HCV. The two types of AFP measurements have different relationship of clinical covariates. We found that serum and tissue AFP were both associated with tumor stage, but serum AFP was associated with tumor size and tissue AFP was associated with tumor number. With the increase of tumor size, serum AFP value was elevated. One literature showed that positive serum AFP was associated with less differentiated tumors, more advanced TNM stage, larger tumor sizes, and inferior survival compared with negative serum AFP (16). These studies remind us that the AFP levels in HCC patients’ sera might be associated with the intrinsic heterogeneities of the HCC tumors. Other reports demonstrated that higher serum AFP (≥10 ng/mL) was associated with higher HCC risk for HCV cure patients and higher post-treatment serum AFP also had a high risk of HCC recurrence for HCV patients (17,18). The different results may be due to the cut-off value of serum AFP.
Furthermore, our study showed that tissue AFP-positive status was associated with poorer OS. Zhu et al. reported that the expression of Oct4, Klf4, Sox2 and c-Myc was significantly higher in AFP-positive tissues compared to AFP-negative tissues due to these markers were closely related to hepatocarcinogenesis (19). Moreover, the expression levels of tissue AFP in metastatic HCCs were lower than that in those HCCs without metastases, but the expression level of tissue AFP showed no significant influence on survival time (8). Our results demonstrated that serum and tissue AFP were not independent factors of OS, but AFP play an important role in HCC progression. The variation trend of serum AFP can be used as an index to evaluate the efficacy for HCC after receiving treatment in previous studies. Serum and tissue AFP for prognosis was affected by other factors, more studies are needed. In a vitro experiment, overexpression of AFP could enhance proliferation, invasion, and migration of HCC cells. Both qPCR and western blot results demonstrated that the expressions of PD-L1, B7-H4, and P65 were significantly higher in the AFP transfection group compared to the controls (20). Further, high levels of acetylated AFP in HCC tissues were associated with HBV infection and correlated with poor prognosis and decreased patient survival, AFP acetylation plays an important role in HCC progression (21).
There are some limitations in the current studies. Firstly, the grouping process were exposed to selection bias due to retrospective study. Secondly, the cut-off value of serum AFP was influenced by different assay kit. Thirdly, a small number of patients were included because of the single-surgeon team, and further lager sample research is needed to clarify the diagnostic value of AFP.
In conclusion, this study showed that serum AFP level could be a highly predictive biomarker for tissue AFP status in patients with HCC. Furthermore, serum AFP levels ≥92.33 ng/mL and tissue AFP-positive status were associated with poorer OS but were not independent factors of OS.
Acknowledgments
Funding: This work was supported by the Capital Health Research and Development of Special Fund Program (No. 2018-1-4021), CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-12M-1-066) and Sanming Project of Medicine in Shenzhen (No. SZSM202011010).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2334/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2334/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2334/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2334/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 19-010/1795), and written informed consents were obtained from all the patients during the hospital stay.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Özdemir F, Baskiran A. The importance of AFP in liver transplantation for HCC. J Gastrointest Cancer 2020;51:1127-32. [Crossref] [PubMed]
- Yang DH, Wang WP, Zhang Q, et al. Hepatocellular carcinoma progression in hepatitis B virus-related cirrhosis patients receiving nucleoside (acid) analogs therapy: A retrospective cross-sectional study. World J Gastroenterol 2021;27:2025-38. [Crossref] [PubMed]
- Chong CC, Lee KF, Ip PC, et al. Pre-operative predictors of post-hepatectomy recurrence of hepatocellular carcinoma: can we predict earlier? Surgeon 2012;10:260-6. [Crossref] [PubMed]
- Murray MJ, Nicholson JC. α-Fetoprotein. Arch Dis Child Educ Pract Ed 2011;96:141-7. [Crossref] [PubMed]
- Zhang H, Qiu C, Zeng H, et al. Upregulation of Stress-Induced Protein Kinase CK1 Delta is associated with a Poor Prognosis for patients with Hepatocellular Carcinoma. Genet Test Mol Biomarkers 2021;25:504-14. [Crossref] [PubMed]
- Jiang Y, Tie C, Wang Y, et al. Upregulation of serum sphingosine (d18:1)-1-P potentially contributes to distinguish HCC including AFP-negative HCC from cirrhosis. Front Oncol 2020;10:1759. [Crossref] [PubMed]
- Wang X, Mao M, He Z, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci 2019;15:221-8. [Crossref] [PubMed]
- Mao R, Zhao JJ, Bi XY, et al. A low neutrophil to lymphocyte ratio before preoperative chemotherapy predicts good outcomes after the resection of colorectal liver metastases. J Gastrointest Surg 2019;23:563-70. [Crossref] [PubMed]
- Zhang C, Zhang J, Wang J, et al. Alpha-fetoprotein accelerates the progression of hepatocellular carcinoma by promoting Bcl-2 gene expression through an RA-RAR signalling pathway. J Cell Mol Med 2020;24:13804-12. [Crossref] [PubMed]
- Tsilimigras DI, Moris D, Hyer JM, et al. Serum α-fetoprotein levels at time of recurrence predict post-recurrence outcomes following resection of hepatocellular carcinoma. Ann Surg Oncol 2021;28:7673-83. [Crossref] [PubMed]
- Park H, Lee Y, Lee K, et al. The Clinicopathological significance of YAP/TAZ expression in hepatocellular carcinoma with relation to hypoxia and stemness. Pathol Oncol Res 2021;27:604600. [Crossref] [PubMed]
- Li TH, Qin C, Zhao BB, et al. Identification METTL18 as a potential prognosis biomarker and associated with immune infiltrates in hepatocellular carcinoma. Front Oncol 2021;11:665192. [Crossref] [PubMed]
- Zhao T, Jia L, Li J, et al. Heterogeneities of site-specific N-glycosylation in HCC tumors with low and high AFP concentrations. Front Oncol 2020;10:496. [Crossref] [PubMed]
- Tanaka Y, Ogawa E, Huang CF, et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int 2020;14:1023-33. [Crossref] [PubMed]
- Watanabe T, Tokumoto Y, Joko K, et al. AFP and eGFR are related to early and late recurrence of HCC following antiviral therapy. BMC Cancer 2021;21:699. [Crossref] [PubMed]
- Zhu M, Li W, Lu Y, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer 2017;140:1346-55. [Crossref] [PubMed]
- Li QT, Qiu MJ, Yang SL, et al. Alpha-fetoprotein regulates the expression of immune-related proteins through the NF-κB (P65) pathway in hepatocellular carcinoma cells. J Oncol 2020;2020:9327512. [Crossref] [PubMed]
- Xue J, Cao Z, Cheng Y, et al. Acetylation of alpha-fetoprotein promotes hepatocellular carcinoma progression. Cancer Lett 2020;471:12-26. [Crossref] [PubMed]


