
The size and sternal involvement of chest wall resections for malignant disease predict postoperative morbidity
Introduction
The surgical management of chest wall tumors is challenging as it must achieve complete resection and ensure reconstruction of the thorax with appropriate stability and function (1,2). Although chest wall resection is not very frequent (3), numerous techniques and materials have emerged over the years to improve reconstruction (4-8). Nowadays, these surgeries are relatively safe with acceptable aesthetic results and good post-operative chest wall function. The resulting quality of life is good despite mutilating surgeries (9).
Oncological situations requiring chest wall resection include lung tumors or breast tumors secondarily invading the chest, primary tumors of the chest (sarcoma, desmoid) and chest wall metastases originating from other locations.
While the resection/reconstruction of chest wall tumors has been widely described in literature, the impact of chest wall resection parameters (i.e., size, location) and of reconstruction type on the postoperative course of patients has often been assessed. It appears evident that chest wall resections can affect the quality/function of the respiratory pump, which can cause atelectasis, pneumonia and related complications. Also, the precise identification of patients at risk for complications could help reinforce pre- and postoperative physical therapy protocols.
We report a single center cohort study of chest wall resections/reconstructions and assess the impact of resection/reconstruction parameters on the postoperative course as well as on the disease-free and overall survival. Because this study focused on postoperative respiratory morbidity, we excluded chest surgeries involving lung resections to avoid confounding factors. The identification of a chest wall resection size cut-off and the role of sternal resection on postoperative complications are discussed. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2143/rc).
Methods
Patients
We reviewed all consecutive patients who underwent chest wall resection and reconstruction performed for tumors invading the thorax in our institution between January 2003 and January 2018. Of the 241 chest wall resections identified, we excluded all patients that had undergone combined chest and lung resection, because most complications were lung infections. We also excluded patients with an etiology other than neoplasia. Finally, we only included patients with chest wall resection that required synthetic material or muscle flaps for reconstruction, thus kept 93 chest wall resections in 88 patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local Ethics Committee of Canton de Vaud (No. 2019-00242). Individual consent was waived due to the retrospective nature of this study. Pre-operative assessment was similar in all patients and included radiological examination by computed tomography (CT)-scan and positron emission tomography (PET)-scan when indicated. An additional magnetic resonance imaging (MRI) exam was carried out if brain metastases had to be ruled out. Functional testing was performed when necessary and included pulmonary function assessment with forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) and a transthoracic echocardiography. Finally, all cases were discussed by our multidisciplinary tumor board to confirm the indication for resection.
Oncological workup
Chest wall resections were separated into three groups: breast tumors invading the chest (Group 1), primary chest wall tumors (soft tissue sarcomas, angiosarcomas, Ewing sarcomas, desmoid tumors, radiation-induced soft tissue sarcomas, Group 2) and chest wall metastasis originating from distant tumors (Group 3). Oncological workup in Groups 1 and 2 involved a chest CT scan, chest MRI and PET-CT. Resections were performed in most cases in the context of a multimodal management protocols (chemotherapy ± radiation therapy + surgery). For Group 3, surgery was considered only in situations of oligometastasis in combination with systemic therapies.
In the postoperative phase, adjuvant treatments were applied when necessary and involved chemotherapy, radiation therapy or surveillance according to the tumor pathology, completeness of resection and loco-regional invasion.
Surgical approach
All surgeries reported in this study were full thickness chest wall resections for which a reconstruction with synthetic material, muscle flap or a combination of both was required. For each patient, we recorded the age, comorbidities, tumor type, location and extent of resection (clavicle, sternal involvement), type of reconstruction, 30-day postoperative complications, long term overall survival, disease-free survival and mortality. Lung atelectasis was defined as an occluded bronchus with distal lung collapse. This condition was reversible with lung physiotherapy, patient mobilization and, in some cases, secretion clearing by bronchoscopy. Lung pneumonia was defined as a lung condensation with an aeric bronchogram, blood inflammation, systemic effects and positive sputum or secretion cultures.
Statistical analysis
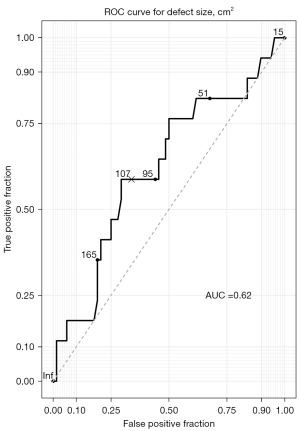
Comparative statistics were performed using Statview and Excel [analysis of variance (ANOVA), Student t-test, Fisher exact when indicated] and Cox regression analysis tests. The survival of each group was estimated with a Kaplan-Meier estimator and censored if a patient was lost during follow-up. The survival distribution of the groups was compared with a log-rank test. Statistical significance threshold was set at P<0.05. P values between 0.10 and 0.05 were reported as “bordering on significant”. In order to compute the value of the defect size which best separates patients with and without complications, a receiver operating characteristic (ROC) curve analysis plotting chest wall resection size versus lung complications (atelectasis and/or pneumonia) is used. The optimal value of defect size is computed using the Youden index (J) method. We determined the area under the curve (AUC) and found a maximal discrimination point at 114 cm2 area with 62% discrimination. Kaplan-Meier survival curves, the ROC curve analysis and uni- or multivariable analyses were obtained using R version 3.5.1, using a generalized linear model (GLM) for the latter analysis. We assessed the impact of sex, age, type of resection and size of chest defect (above or below 114 cm2) and surgery duration (above or below the average 248 min) on post-operative complications. Because few tests were performed, we did not carry out any adjustment for multiple testing. The P values we report may not be interpreted as confirmatory but are descriptive in nature and inferences drawn from the 95% confidence intervals (CIs) may not be reproducible.
Results
Patients
During the 15-year study period, our surgical team performed 93 chest wall resections in 88 patients. The 5 additional chest wall resections performed in the same patients were required because of local tumor recurrence. There were 36 males and 52 females with a mean age of 56±17 (range, 17–91) years at the time of surgery. Co-morbidities involved a prior history of cancer that preceded the chest wall neoplasm (n=63, 72%), smoking (n=22, 25%), hypertension (n=32, 36%) and diabetes (n=14,16%). Patient characteristics are summarized in Table 1. The oncological indications for chest wall resection were breast cancers with chest wall invasion (Group 1, n=21, ductal and lobular carcinoma), primary malignant chest wall tumors (Group 2, n=45, soft tissue sarcomas, angiosarcomas, Ewing sarcomas, desmoid tumors, radiation-induced soft tissue sarcomas), and chest wall metastasis occurring from controlled distant neoplasms (Group 3, n=27, mostly breast, sarcoma, renal and cancers of the ears nose and throat).
Table 1
| Characteristics | Value |
|---|---|
| Total population | 88 |
| Sex, n [%] | |
| Female | 52 [59] |
| Male | 36 [41] |
| Age (years), mean ± SD | 56±17 |
| Comorbidities, n [%] | |
| Cardiopathy | 9 [10] |
| HBP | 32 [36] |
| Tobacco exposure | 22 [25] |
| COPD | 5 [6] |
| Obesity | 10 [11] |
| DM | 14 [16] |
| Alcohol abuse | 2 [2] |
| Previous cancer | 63 [72] |
| Pulmonary functions, mean ± SD | |
| % Predicted FEV1 | 92%±22% |
| % Predicted DLCO | 77%±18% |
SD corresponds to the standard deviation of the results. SD, standard deviation; HBP, high blood pressure; COPD, chronic obstructive pulmonary disease (defined as FEV1 <60%); DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lung for carbon monoxide.
Operative details of chest wall resection
The surgical procedure was adapted for each group: in Group 1, chest wall resection was associated to additional mastectomy when necessary and to axillary lymph node dissection when indicated; in Group 2, wide en-bloc resection of the chest wall with the lining muscle compartments and skin-sparing was realized whenever possible; in Group 3, a complete resection of the chest wall was carried out with associated lining structures whenever necessary. The average duration of the intervention was 248±126 (range, 96–910) minutes. Chest wall resection characteristics are reported in Table 2. Thoracic resections involved ribs (57%) alone, or a combination of ribs ± sternum ± clavicle (43%). Chest wall resections consisted in bony and intercostal muscle resection alone (n=38, 41%) or full thickness resections involving superficial muscles, subcutaneous tissue and skin (n=55, 59%). Most chest wall resections were located in the anterior (57% of all resections) upper portion of the chest (the main location of the chest resection was on rib 1 to 5, 70%). The average bony defect size was 107±80 (range, 15–375) cm2 and average cutaneous defect size was 88±134 (range, 0–650) cm2. Anatomopathological reports were available for 92 of the 93 cases. Complete tumor resection was achieved in 66 cases (72%) and 26 cases had evidence of microscopic R1 resection (28%, mostly in Group 1). There was no case of macroscopic R2 invasion.
Table 2
| Type and location of chest wall resection | Number of patients [%] |
|---|---|
| Resection | |
| Ribs | 53 [57] |
| Resected ribs (ribs), mean ± SD | 3±2 |
| Sternum + ribs | 21 [23] |
| Sternum + ribs + clavicle | 11 [12] |
| Sternum | 2 [2] |
| Clavicle | 2 [2] |
| Sternum + clavicle | 2 [2] |
| Ribs + clavicle | 2 [2] |
| Location | |
| Upper chest (ribs 1–5) | 70 [75] |
| Lower chest (ribs 6–12) | 23 [25] |
| Anterior | 53 [57] |
| Posterior | 11 [12] |
| Lateral | 29 [31] |
SD, standard deviation.
Operative approaches to reconstruction
All patients included in our study underwent chest wall reconstruction with either replacement of the bony chest wall by synthetic material (mesh and/or osteosynthesis) (n=23, 25%), muscle flap mobilization (n=13, 14%), or a combination of both (n=57, 61%). Bony chest wall reconstruction was achieved with synthetic material (80 cases, 86%) using meshes alone (Vicryl/Mersilene, n=21), osteosynthesis material alone (n=2), or a combination of both associated with flaps (n=57). Synthetic meshes were folded back on themselves to ensure better resistance and were sandwiched by methylmetacrylate in 44% of these 80 cases. In the remaining 13 cases, utilization of synthetic material seemed too hazardous (post radiation therapy/post infection). For these, we used the muscle flap fascia as an alternative to the mesh for chest wall reconstruction. A total of 72 flaps (n=66, 92% pedicled and n=6, 8% free) were used to cover synthetic material or replace missing tissue. For pedicled flaps, we used mainly latissimus dorsi (n=60), serratus anterior (n=4) and pectoralis major (n=2). Free flaps consisted in anterolateral thigh (n=5) and deep inferior epigastric perforator flaps (n=1). A skin paddle was collected on the flaps in 52 cases to replace missing tissue following resection. Additional coverage by mesh grafts was achieved in 10 cases. A representative case of resection/reconstruction using a pedicled muscle flap is presented in Figure 1.
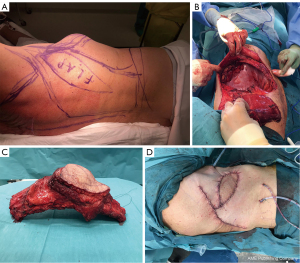
In-hospital morbidity and mortality
The main complications experienced during hospital stay were pneumonia (n=15, 16%), atelectasis (n=6, 6%) arrhythmia (atrial fibrillation, n=6, 6%) and pleural effusion/hemothorax (n=15, 16%). Re-intervention (Clavien-Dindo IIIB) was required in 12 patients (13%) essentially to manage chest wall or thoracic hematomas (5 cases), hemothorax evacuation (5 cases) or for flap revisions (2 cases including one significant necrosis requiring its removal and replacement with a rotation flap). Operators most often treated pneumonia patients with antibiotherapy, recurrent bronchoscopies and intensive respiratory physiotherapy. Among our pneumonia cases, only two patients had parapneumonic effusions requiring drainage and three patients with atelectasis developed pneumonia. One patient’s condition evolved to a lethal septic shock and one patient required ventilatory support in the intensive care unit (ICU) which resulted in a percutaneous tracheotomy. The majority of our patients were transferred directly to our department postoperatively. Twelve patients required a short stay (<4 days) in the ICU for postoperative monitoring. None of them required prolonged ventilatory support.
Four cases required longer stays (>4 days). There were 3 deaths in the 30-day postoperative period (3%): 2 multiorgan failures (1 following a myocardial infarct and 1 septic shock of pulmonary origin) and 1 hemorrhagic shock due to a tear in the ascending aorta caused by a sternal fragment displacement. Complications are summarized in Table 3. A total of 43 patients (46%) experienced at least one complication during their stay. Hospital length of stay (LOS) was 16±10 days. In order assess the correlation between defect size and pulmonary complications, we plotted the defect size against these complications in a ROC curve (Figure 2). We determined the AUC and found a maximal discrimination point at 114 cm2 area with 62% AUC, which implies that defect size is a good discriminant for pneumonia or atelectasis.
Table 3
| Type of complication | Number of patients (%) |
|---|---|
| Systemic complications | |
| Pneumonia | 15 (16%) |
| Atelectasis | 6 (6%) |
| Arrythmia | 6 (6%) |
| Multiorgan failure | 2 (2%)# |
| Massive hemorrhage | 1 (1%)# |
| Local complications | |
| Pleural effusion/hemothorax | 15 (16%) |
| Seroma | 5 (7%)* |
| Neuropraxia (recurrent nerve/brachial plexus) | 8 (2/6) (9%) |
| Partial loss of the flap | 3 (4%)* |
| Total loss of the flap | 1 (1%)* |
| Wound dehiscence | 4 (4%) |
| Synthetic material infection | 1 (1%)** |
#, leading to patient death; * calculated on the total number of flaps (n=72); ** calculated in relation to the total number of synthetic materials (n=80).
To assess the impact of resection parameters on postoperative complications, we analyzed how age, gender, chest defect size, location, partial/complete thickness, induction therapy and reconstruction type were associated with postoperative complications by uni- and multivariable analysis (Tables 4,5). In both instances, we found that bone defect size above 114 cm2 was associated with increased pneumonia development [odds ratio (OR) =4.29, 95% CI: 1.32–15.41, P=0.02 on univariable and OR =3.67, 95% CI: 1.01–14.63, P=0.05 on multivariable analysis]. Similarly, chest wall resection involving the sternum was significantly associated to postoperative atelectasis (OR =8.75, 95% CI: 1.57–67.21, P=0.02 on univariable and OR =78.92, 95% CI: 4.01–9,005.94, P=0.02 on multivariable analysis).
Table 4
| Parameters | OR | 95% CI | P value |
|---|---|---|---|
| Pneumonia | |||
| Female gender | 0.36 | 0.11–1.10 | 0.08 |
| Age >56 years | 1.39 | 0.46–4.49 | 0.57 |
| Operation duration (>248 min) | 0.85 | 0.24–2.72 | 0.79 |
| Type of cancer | |||
| Breast cancer | 0.20 | 0.01–1.12 | 0.14 |
| Chest wall | 1.71 | 0.56–5.54 | 0.35 |
| Metastasis | 1.33 | 0.38–4.23 | 0.64 |
| Type of resection | |||
| >3 ribs | 2.17 | 0.68–8.40 | 0.22 |
| Ribs | 0.86 | 0.28–2.67 | 0.79 |
| Sternum | <0.01 | NA | 0.99 |
| Sternum + ribs | 1.39 | 0.35–4.69 | 0.61 |
| Clavicle | <0.01 | NA | 0.99 |
| Full thickness | 1.07 | 0.35–3.46 | 0.91 |
| Bone defect >114 cm2 | 4.29 | 1.32–15.41 | 0.02 |
| Skin defect >88 cm2 | 2.10 | 0.62–7.05 | 0.22 |
| Location | |||
| Upper chest | 2.44 | 0.60–16.43 | 0.27 |
| Lower chest | 0.41 | 0.06–1.65 | 0.27 |
| Anterior | 1.22 | 0.34–5.78 | 0.78 |
| Posterior | 0.88 | 0.19–3.18 | 0.86 |
| Type of reconstruction | |||
| Flap | 0.39 | 0.02–2.22 | 0.38 |
| MatrixRib | <0.01 | NA | 0.99 |
| Mesh + flap | 1.50 | 0.48–5.20 | 0.50 |
| Atelectasis | |||
| Female gender | 0.11 | 0.01–0.74 | 0.0051 |
| Age >56 years | 1.82 | 0.34–13.64 | 0.50 |
| Operation duration (>248 min) | 0.38 | 0.02–2.67 | 0.39 |
| Type of cancer | |||
| Breast cancer | <0.01 | NA | 0.99 |
| Chest wall | 5.75 | 0.88–112.63 | 0.12 |
| Metastasis | 0.49 | 0.02–3.23 | 0.52 |
| Type of resection | |||
| >3 ribs | 3.78 | 0.58–73.97 | 0.23 |
| Ribs | 0.36 | 0.05–1.95 | 0.25 |
| Sternum | <0.01 | NA | 0.99 |
| Sternum + ribs | 8.75 | 1.57–67.21 | 0.02 |
| Clavicle | <0.01 | NA | 0.99 |
| Full thickness | 0.33 | 0.04–1.77 | 0.21 |
| Bone defect >114 cm2 | 0.91 | 0.12–4.97 | 0.92 |
| Skin defect >88 cm2 | 0.53 | 0.03–3.78 | 0.58 |
| Location | |||
| Upper chest | >100 | NA | 0.99 |
| Lower chest | <0.01 | NA | 0.99 |
| Anterior | 0.57 | 0.10–4.31 | 0.53 |
| Posterior | 1.89 | 0.25–10.51 | 0.48 |
| Type of reconstruction | |||
| Flap | <0.01 | NA | 0.99 |
| MatrixRib | <0.01 | NA | 0.99 |
| Mesh + flap | 0.69 | 0.12–3.89 | 0.66 |
OR, odds ratio; CI, confidence interval; NA, not available (group too small for meaningful analysis).
Table 5
| Parameters | OR | 95% CI | P value |
|---|---|---|---|
| Pneumonia | |||
| Female gender | 0.41 | 0.11–1.45 | 0.17 |
| Age (per additional year of age) | 1.02 | 0.99–1.07 | 0.24 |
| Operation duration (>248 min) | 0.80 | 0.20–2.99 | 0.74 |
| Type of resection | |||
| Bone defect >114 cm2 | 3.67 | 1.01–14.63 | 0.05 |
| Atelectasis | |||
| Female gender | 0.04 | 0.01–0.51 | 0.04 |
| Age (per additional year of age) | 1.14 | 1.02–1.41 | 0.08 |
| Operation duration (>248 min) | 0.21 | 0.01–3.65 | 0.36 |
| Type of resection | |||
| Sternum | 78.92 | 4.01–9,005.94 | 0.02 |
| Bone defect >114 cm2 | 0.12 | 0.01–1.81 | 0.20 |
OR, odds ratio; CI, confidence interval.
Long term follow-up
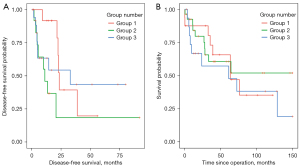
Following discharge, late surgical complications were rare and consisted of reconstruction material infection (n=5, mesh, methylmetacrylate or both) that were managed surgically by replacing synthetic material or by a resorbable or biocompatible mesh. Other long-term complications included partial necrosis of the flap managed by debridement (n=2) and seroma formation (n=4). Surgery-related chronic pain rate was 8% (n=7). All patients underwent oncological surveillance according to the established international guidelines [European Society for Medical Oncology (ESMO)]. Mean follow-up was of 46±44 months. Cancer recurrence occurred in 47% (n=44) of patients and mostly consisted of distant metastases (n=39). Local recurrence accounted for 16% (n=15) of all cases. Recurrences were managed by chemotherapy, and/or radiation therapy and/or surgery. Disease-free and overall survival were 37±43 and 48±42 months for primary malignancy and of 24±33 and 48±53 months for metastatic chest wall tumors respectively. These results must be balanced by the fact that they are based on subpopulations of patients with a complete record (n=59 patients for overall survival and n=44 patients for disease-free survival). The 5-year overall survival was 44% in Group 1, 54% in Group 2, and 46% in Group 3 (Figure 3).
Discussion
Oncological results
Chest wall resection and reconstruction are a validated approach to manage thoracic wall malignancies. Completeness of resection and appropriate reconstruction are necessary for optimal short- and long-term outcomes (3,10). Oncologically, the approaches have evolved over the past years with the advent of PET-CT, which allows a more precise staging of the oncological disease and the improvements in neo-adjuvant/adjuvant therapies. Surgical techniques have also evolved with osteosynthesis and replacement material (11) that have allowed to push back the limits of resection completeness (R0). In this study, we distinguished 3 separate groups, based on the underlying oncological disease: Group 1: breast cancers secondarily invading the chest; Group 2: primary malignant chest wall tumors; and Group 3: chest wall metastases. Breast cancers invading the chest wall can be of two subtypes: initial presentation of a breast tumor with chest wall involvement or breast tumor recurrence with chest wall involvement. In our study, all situations consisted of breast cancer recurrences with a small fraction that also had axillary lymph node invasion (N). Their 5-year survival was 44%. Patient selection as well as associated therapies are essential for optimal survival results (12). Regarding primary chest wall tumors invading the chest, most cases in this study were sarcomas. It has been advocated to perform at least 2 to 4 cm tumor-free margins (13) which can be difficult to achieve depending on the location of the tumor. Most cases were managed by surgery upfront (no induction chemo or radiation therapy). Neoadjuvant chemotherapy or radiation therapy were only performed in 31% of patients (n=14/45). This is likely related to the fact that most were accessible sarcomas that could be resected with good margins, thus avoiding pre-operative radiation therapy which is toxic for the underlining lung tissue. Completeness of resection was achieved in 69% (n=31/45) of cases with overall survivals similar to previously published studies (10,14). Finally, chest resection approaches were also performed in the field of thorax metastases. In this particular group, care was taken to ensure all primary locations of the tumor were controlled. Patients obtained good long-term survival (54% at 5 years) which could reflect the importance of patient selection for which limited experience exists in the literature (15-17).
Chest wall reconstruction
The relevance of reconstruction of the bony chest wall is still debated, especially in cases of minor chest wall resections. Most authors agree that chest wall defects involving more than four ribs or a defect greater than 5cm in diameter (equivalent to about 20 cm2) should be reconstructed to prevent paradoxical chest movement (18). Based on the literature, only 40% to 50% of chest wall resections require subsequent reconstruction (10). The average size of the chest wall defects in our study was 107±80 cm2 and required reconstruction in most cases. Given the fact the study took place over many years, our surgical practice evolved. Our department published a retrospective review (19) which compared pre-operative to 6-month postoperative FEV1 combined to chest MRI to diagnose paradoxal movements. Pulmonary functions were maintained and paradoxal movement was only observed in 8% of all reconstructions. Despite these results, we switched over time from synthetic meshes covered with methymetacrylate to multilayered meshes associated to anatomical osteosynthesis material mostly because of infection risks of the methymetacrylate (6 infections of which 5 on methymetacrylate material). Reconstruction materials have different resistance, rigidity and anatomical restauration properties. Irrespective of the material used, we did not experience material failure (osteosynthesis rupture or mesh tears).
Different reconstruction approaches and material are possible dependent on the center/surgeon’s experience. For complex resections/reconstruction with important bony/soft tissue defects, operators planned all surgeries with a multidisciplinary team of thoracic and plastic/reconstructive surgeons. This approach was useful to choose the optimal muscle flap and in deciding if free flaps were necessary (as well as where to establish anastomoses). Flap results were excellent with only 5 cases that required minor revision that could be handled by debridement or conservative treatment and one case with total flap necrosis needing removal and replacement by another flap.
Factors affecting postoperative complications
Very few studies have assessed the impact of chest wall resection size or location on postoperative patient morbidity. It has been reported that extensive bony and soft tissue resections were associated with more secretion retention and chest wall instability (flail chest) leading to prolonged hospital stay (20). This did not seem to affect the quality of life on the long run. Surgery duration and resection extent were shown to affect postoperative complications such as deep venous thrombosis, pulmonary embolism and cardiac arrest (20,21). Using ROC analysis, we determined that the cutoff chest wall defect for optimal association with the occurrence of complications was 114 cm2. Our uni- and multivariable analysis demonstrate that defects greater than 114 cm2 were significantly associated to pneumonia development. Similarly, sternal resection was significantly associated with the occurrence of atelectasis. Interestingly, procedure duration, type of reconstruction and location of the resection had no impact on patient outcome. In addition, as shown previously, a complex perioperative course had no impact on patient survival in the majority of cases. This is likely explained by the active and early measures we introduced to avoid complications in these patients: early mobilization, active spirometry, and secretion evacuation (spontaneous or through bronchoscopies) in the immediate post-operative phase. Recent enhanced recovery after surgery (ERAS) guidelines (22) have helped us better understand the critical points for optimal patient outcomes, including elements such as early mobilization, which have become standard in our institution. Our results therefore suggest that particular care should be given to patients with >114 cm2 resection sizes or resections involving the sternum to limit postoperative morbidity.
Limitations of the study
The main limitation of our study is the retrospective, single center design, which impairs generalization of the results. This is compounded by the fact that several thoracic and reconstructive surgeons were involved in the study. Although every effort was made to standardize the protocols, it is inevitable that some inter-operator variations would occur. Another limitation is the relatively small size and heterogeneity of the groups studied. Whilst it appeared important to stratify by primary tumor type, we recognize that some of the sub-groups are too small to draw any statistically significant conclusion. This should not obfuscate our general observations, only add a degree of caution when interpreting the results of the subgroups. Finally, the patient inclusion spans a 15-year-long interval. During this period, there have been some innovations in operative techniques, reconstructive material science and general patient management, which might have affected the experience that patients underwent. This is not so acute that the overall results of a given operation would be drastically different, but this should be borne in mind. In spite of the above, we did not stratify our analyses by periods within the time of study because this would have resulted in even smaller sub-groups.
Conclusions
In conclusion, chest wall resection/reconstruction is a valid therapeutic approach for chest tumors with interesting results. The reconstruction of chest wall resection is controversial but combined approaches rooted in the center’s experience and multidisciplinary teams have helped improve this element. With prompt and adequate post-operative management, this type of surgery can be performed with minimal complications, including major resections. Particular care should be given to patients with resection sizes above a certain threshold (114 cm2 in our dataset) or involving the sternum to minimize the risk of pneumonia/atelectasis.
Acknowledgments
We wish to acknowledge help with the statistical analysis provided by Mr. Gilles Kratzer, PhD.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2143/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2143/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2143/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local Ethics Committee of Canton de Vaud (No. 2019-00242). Individual consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Rocco G. Chest wall resection and reconstruction according to the principles of biomimesis. Semin Thorac Cardiovasc Surg 2011;23:307-13. [Crossref] [PubMed]
- Downey RJ, Martini N, Rusch VW, et al. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999;68:188-93. [Crossref] [PubMed]
- Rocco G. Anterior chest wall resection and reconstruction. Oper Tech Thorac Cardiovasc Surg 2013;18:32-41. [Crossref]
- Moradiellos J, Amor S, Córdoba M, et al. Functional Chest Wall Reconstruction With a Biomechanical Three-Dimensionally Printed Implant. Ann Thorac Surg 2017;103:e389-91. [Crossref] [PubMed]
- Berthet JP, Wihlm JM, Canaud L, et al. The combination of polytetrafluoroethylene mesh and titanium rib implants: an innovative process for reconstructing large full thickness chest wall defects. Eur J Cardiothorac Surg 2012;42:444-53. [Crossref] [PubMed]
- Betancourt Cuellar SL, Heller L, Palacio DP, et al. Intra- and Extra-Thoracic Muscle Flaps and Chest Wall Reconstruction Following Resection of Thoracic Tumors. Semin Ultrasound CT MR 2017;38:604-15. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Heuker D, Lengele B, Delecluse V, et al. Subjective and objective assessment of quality of life after chest wall resection. Eur J Cardiothorac Surg 2011;39:102-8. [Crossref] [PubMed]
- Scarnecchia E, Liparulo V, Capozzi R, et al. Chest wall resection and reconstruction for tumors: analysis of oncological and functional outcome. J Thorac Dis 2018;10:S1855-63. [Crossref] [PubMed]
- Demondion P, Mercier O, Kolb F, et al. Sternal replacement with a custom-made titanium plate after resection of a solitary breast cancer metastasis. Interact Cardiovasc Thorac Surg 2014;18:145-7. [Crossref] [PubMed]
- Sepesi B. Management of Breast Cancer Invading Chest Wall. Thorac Surg Clin 2017;27:159-63. [Crossref] [PubMed]
- Yoon SH, Jung JC, Park IK, et al. Clinical Outcomes of Surgical Treatment for Primary Chest Wall Soft Tissue Sarcoma. Korean J Thorac Cardiovasc Surg 2019;52:148-54. [Crossref] [PubMed]
- Abdel Rahman ARM, Rahouma M, Gaafar R, et al. Contributing factors to the outcome of primary malignant chest wall tumors. J Thorac Dis 2017;9:5184-93. [Crossref] [PubMed]
- Hemmati SH, Correa AM, Walsh GL, et al. The prognostic factors of chest wall metastasis resection. Eur J Cardiothorac Surg 2011;40:328-33. [Crossref] [PubMed]
- Warzelhan J, Stoelben E, Imdahl A, et al. Results in surgery for primary and metastatic chest wall tumors. Eur J Cardiothorac Surg 2001;19:584-8. [Crossref] [PubMed]
- McCormack P, Bains MS, Beattie EJ Jr, et al. New trends in skeletal reconstruction after resection of chest wall tumors. Ann Thorac Surg 1981;31:45-52. [Crossref] [PubMed]
- Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis 2016;8:S863-71. [Crossref] [PubMed]
- Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Hazel K, Weyant MJ. Chest Wall Resection and Reconstruction: Management of Complications. Thorac Surg Clin 2015;25:517-21. [Crossref] [PubMed]
- Hardy KL, Davis KE, Constantine RS, et al. The impact of operative time on complications after plastic surgery: a multivariate regression analysis of 1753 cases. Aesthet Surg J 2014;34:614-22. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]


