
Multimodality treatment for multiple recurrences of cervical cancer after radiotherapy: a case report
Introduction
The common treatment for locally advanced cervical cancer is radical concurrent chemoradiotherapy (CCRT); however, even with standard treatment, recurrence is common (1). Over 80% of recurrences occur within 2 years, and the median overall survival (OS) of these patients is less than 12 months, with a 5-year OS of only 1% in untreated patients (1). Treatment of recurrent cervical cancer (RCC) is challenging, although several treatments, including surgery, chemotherapy, radiotherapy, targeted therapy, and emerging immunotherapy, have been assessed. The treatment regimen must be individualised according to the primary treatment, site and extent of recurrence (local, regional, and/or distant metastases), disease-free interval, and general condition of the patient (1,2).
In cases of central pelvic recurrence after failed radiotherapy, combined with bladder and/or rectal invasion that neither extends into the pelvic wall nor shows any evidence of extra-pelvic metastases, pelvic exenteration (PE) with adequate surgical margins is the standard treatment (2). However, owing to increased tissue and vascular fragility after radiotherapy, the postoperative complication rate ranges from 30% to 70%, the most common complications being infection, bowel obstruction, ureteral damage, and, most seriously, vaginal rectal/bladder fistula (3). Some authors recommend PE for recurrences that respond to neoadjuvant chemotherapy (1). However, few relevant studies have been conducted. For cases with pelvic wall invasion or failure to achieve R0 resection, intraoperative radiotherapy (IORT), a single administration of high-dose irradiation to the tumour bed, provides a 1-year local control (LC) of up to 58%, while OS is significantly correlated with the positive margins, with a median survival of 10–17 months (4). Several studies show IORT provide longer LC, but does not longer OS (5). IORT requires advanced equipment and experienced physicians, and there is no uniform paradigm, which limits its broader application. Höckel et al. (6) proposed that laterally extended endopelvic resection is effective for pelvic wall invasion. Although some studies have demonstrated the safety and feasibility of re-external beam radiotherapy (EBRT), it is still not common because of organs at risk (OARs) limitations and potential radiation resistance (7). Therefore, modalities with steep marginal dose curves, such as brachytherapy (BT) and stereotactic body radiation therapy (SBRT), are preferred. BT, which delivers high doses of radiation directly to the tumour, mainly consists of interstitial brachytherapy (ISBT) and radioactive seed implantation. ISBT provides comparable benefits to PE, with reported grade ≥3 complications ranging from 20% to 51% (8-10). ISBT has great potential in pain relief, and 1-year OS of 20% to 52% (11-13). However, comparative studies between ISBT and seed implantation are lacking. Targeted therapy is promising, but response rates to chemotherapy is low (14). Although re-recurrence after salvage treatment is common, few reports describe multimodality therapy and outcomes. This study reports on the experience of treatment after multiple relapses to provide a reference for clinicians. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2250/rc).
Case presentation
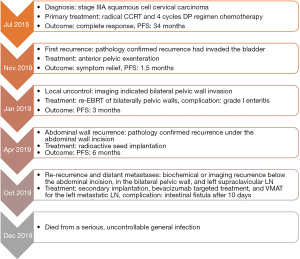
The patient was a 57-year-old woman (gravida 4/para 1) who had no family history other than her father’s history of lung cancer. She was diagnosed with stage IIIA squamous cell cervical carcinoma (Federation International of Gynecology and Obstetrics 2008 staging) in July 2015. From 28 July to 7 September 2015, the whole pelvis was treated with intensity-modulated radiotherapy (IMRT) at a total dose of 5,040 cGy in 28 fractions. Five cycles of weekly cisplatin (40 mg/m2) were administered concurrently. Computed tomography (CT)-guided ISBT was performed in five fractions, twice a week. The treatment process was described previously (15). The high-risk clinical target volume was defined as the entire cervix and local infiltrate volume. The minimum dose delivered to 90% of the target volume was 9,046 cGy. The biological doses equivalent to conventional 2-Gy fractionated radiation administered to the rectum, bladder, and small bowel were 8,259, 9,076, and 7,692 cGy, respectively. Four cycles of chemotherapy (docetaxel 75 mg/m2 and cisplatin 75 mg/m2 every 21 days) were administered. Pelvic magnetic resonance imaging (MRI) 4 months later showed a complete response of the cervical lesions. No biochemical or imaging recurrences were observed on regular follow-up thereafter. The last follow-up was conducted on 28 August 2017.
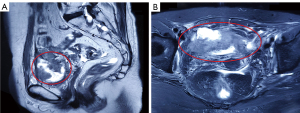
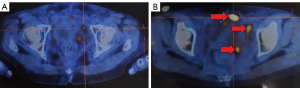
On 5 November 2018 (34 months after the last cycle of chemotherapy), recurrent disease invading the bladder was confirmed by pathology and imaging (Figure 1A,1B), at which point the patient had experienced 7 months of carnal haematuria. Gynaecological examination revealed a giant cervical lesion measuring approximately 3 cm × 5 cm with bilateral invasion of the pelvic walls. Laboratory examination revealed moderate anaemia. No distant metastases were observed on chest CT. PE was the first choice for recurrence in the radiation field. No intestinal invasion was observed during intraoperative exploration. Anterior PE, which included total hysterectomy, bilateral adnexectomy, vaginectomy, total cystectomy, urethrectomy, bilateral partial ureterectomy, left pelvic lymphadenectomy followed by bilateral ureteral stent placement, percutaneous ureterostomy, and pelvic floor closure, was performed under general anaesthesia on 27 November 2018. Postoperative pathology indicated moderately differentiated keratinised squamous cell carcinoma of cervical origin invading the entire posterior bladder wall, measuring 7.5 cm × 5 cm × 2.2 cm, with numerous vessel cancer emboli, fibrofatty tissue cancer infiltration, nerve invasion, positive bilateral pelvic wall incision margins, and left pelvic lymph node (LN) metastasis (1/1). Postoperative healing of the incision was delayed by 1 month.
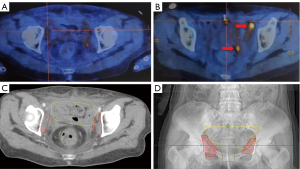
Positron emission tomography (PET)-CT on 23 January 2019 showed bilateral localised invasion of the pelvic walls (Figure 2A,2B). Due to the scattered and deep location of the small lesions, a pathological biopsy specimen could not be taken, but the patient agreed to be treated according to the principles of recurrence. Based on postoperative pathology and consultation with the surgeon and radiologist, the target volume of reirradiation was determined to be the bilateral pelvic walls (Figure 2C,2D). Volumetric modulated arc therapy (VMAT) with a total dose of 3,000 cGy was administered from 24 January to 24 February 2019 in 15 fractions, five times per week (maximum intestinal spot dose: 3,196 cGy). During radiotherapy, the patient developed grade I radiation enteritis (16), which was relieved by symptomatic treatment.
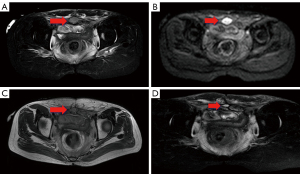
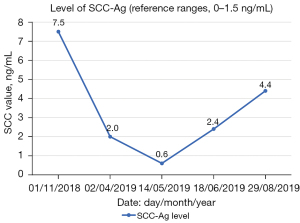
A metastasis under the abdominal incision was diagnosed on imaging and pathology 4.5 months postoperatively (Figure 3A,3B). Twenty-nine radioactive seeds of 125I with an activity of 0.6 were implanted in the isolated lesion after bowel preparation on 12 April 2019. Pelvic MRI at 1 and 4.5 months after implantation indicated that the lesion was stable (Figure 3C,3D); however, there was a gradual increase in squamous cell carcinoma antigen (Figure 4).
PET-CT on 17 October 2019 detected high 18F-fluorodeoxyglucose (FDG) uptake below the previous incision, in the bilateral pelvic wall, and in the left supraclavicular LN (Figure 5A,5B). Inflammatory disease could also lead to high FDG concentrations and was not a diagnostic criterion for recurrence and metastasis. However, the patient refused pathological biopsy and all SUVmax values exceeded 2.5, making it highly likely that the lesion was malignant. She agreed to intervention based on malignancy management. As she was too frail to tolerate chemotherapy, a total dose of 5,750 cGy VMAT was administered to the left metastatic supraclavicular LN starting on 28 October 2019 in 25 fractions, five times per week (Figure 6A,6B). The following day, she received 400 mg of bevacizumab intravenously and underwent a second implantation of 24 125I seeds with an activity of 0.6 at the hypermetabolic lesion under the abdominal wall.
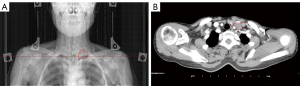
No complications occurred during treatment. However, when the cumulative dose received by the left supraclavicular LN reached 2,070 cGy (10 days after bevacizumab and 4 days after the second seed implantation), radiotherapy to the supraclavicular region was ceased because of the development of an intestinal fistula in the abdominal wall surgical scar (Figure 7). The patient died on 28 December 2019 due to severe general infection. She had an OS of 47 months, a progression-free survival (PFS) of 34 months after the last chemotherapy, and a post-relapse survival of 13.5 months. The treatment process is shown in Figure 8.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The treatment of RCC, especially multiple recurrences, is challenging and we report one such case and share our experiences. In this case, the initial recurrence was a central recurrence within the primary radiation field. The treatment was limited because of the bulky lesion (maximum diameter, 7.5 cm), history of radiotherapy, and severe clinical symptoms (carnal haematuria and anaemia). In patients with recurrences limited to the pelvis, including invasion of the pelvic organs (bladder and/or rectum), and no invasion of the pelvic sidewall (the typical clinical triad is unilateral lymphedema, hydronephrosis, and sciatica) or distant metastases, PE is the first choice if negative margins can be secured (3). The 5-year OS is over 30%, but the postoperative complication rate is high because of increased tissue and vessel fragility (17). Although clinical examinations showed pelvic wall invasion, we had no choice but to perform a cautious surgery. Intraoperative exploration did not reveal any intestinal invasion; therefore, an anterior PE with resection of the genital tract and bladder only was performed. Nevertheless, the ultimate cause of death was a serious, general, and uncontrollable infection caused by an intestinal fistula. The development of a fistula might not affect survival if total PE or prophylactic colostomy are performed.
Despite many studies confirming that PE results in a favourable LC, the recurrence rate after PE is 60% (3). Important prognostic factors are recurrent interval, tumour size, pelvic wall invasion, positive surgical margins, and lymphovascular invasion (2,17). The patient’s postoperative pathology predicted a poor prognosis. When a lesion invades the pelvic wall and there are no distant metastases, especially in patients with residual macroscopic or microscopic tumours, IORT does not increase OS but results in longer LC (5). A longer treatment interval is beneficial in improving patient quality of life and reducing serious toxicities such as fistulas (10). It can also reduce the risk of implanted metastasis. However, the hospital where our patient’s surgery was performed was not equipped for IORT. Further, her other serious symptoms did not allow for additional treatment, and the surgical incision took 1 month to heal, even without additional treatment. Although there were several subsequent recurrences, it is undeniable that PE improved her symptoms and quality of life.
At 1.5 months postoperatively, small and scattered hypermetabolic lesions were found bilaterally in the pelvic wall. As no postoperative examination was performed, it was difficult to determine whether these lesions were recurrent or residual. We performed EBRT because of the small, scattered, and deep pelvic lesions. Some studies have demonstrated the safety and feasibility of EBRT in combination with IORT and BT (7). In a study of treatment regimens including 260 patients with RCC, patients receiving radiotherapy had better PFS and OS than patients receiving other regimens, although this was observed for those with pelvic recurrence only and it was not equally represented in the re-irradiation subgroup (18). Further, determining the appropriate dose and volume remains a serious issue. Tumour control is positively correlated with irradiation dose, but the OARs must be taken into account. The low dose administered here was associated with nearly 8 months’ LC in the pelvic wall and only grade I toxicity, but distant metastasis was not controlled.
Recurrence under the abdominal incision was observed at 4.5 months after surgery, although this could have been implanted metastasis. The patient was treated with surgery and radiotherapy within a short time, and the incision had healed too poorly to tolerate surgery again. The cumulative dose limitation to the OARs remains an important issue. The dosimetric characteristics of ISBT allow it to maximise the target irradiation dose while minimising the OARs dose. The superficial location of the individual lesion also made the implementation of ISBT feasible and convenient. Mahantshetty et al. (9) administered ISBT to centric recurrence within the pelvic irradiated field and showed a complete response rate (CRR) of 76%, with 2-year LC and OS rates of 44% and 52%, respectively. No fistulas occurred as a result of re-irradiation (9). A similar study by Mabuchi et al. (10) achieved a CRR of 60%, a median post-recurrence survival period of 32 months, and a 5-year OS of 52.6%. However, the incidence of fistulas was 20% (10). Repeated injections are required to achieve a high prescribed dose, but this increases the risk of fistula, especially if accompanied by a history of poor incision healing. Another option is radioactive seed implantation, which distributes radioactive seeds within the target lesion, thus providing direct and continuous irradiation. Tong et al. (13) performed CT-guided implantations in 33 patients with pelvic recurrence after radiotherapy and achieved not only an 18-month LC of 33.3%, median local tumour PFS of 7 months, and 1- and 2-year OS of 65.5% and 43.6%, respectively, but also pain relief in over 80% of patients. Moreover, only one case of vaginorectal fistula was observed. SBRT is another potential option. In a study of SBRT in RCC, the 2-year LC and OS were 82.5% and 57.5%, respectively. Further, complications were mild, although they were more serious among patients who had received primary pelvic radiotherapy (19). Both BT and SBRT have therapeutic advantages for oligometastases. BT is applied more widely and is more suitable for superficial lesions, whereas SBRT is mainly performed for oligo-metastases, such as LN metastases. In this case, local disease was evaluated as stable at 1, 2 and 4.5 months after implantation.
We administered salvage bevacizumab for recurrence and metastases 6 months after seed implantation, and a secondary seed implantation was performed in the abdominal wall recurrence. The GOG 240 trial found that bevacizumab, an anti-vascular endothelial growth factor inhibitor, achieves good PFS and OS in combination with chemotherapeutic agents in recurrent and metastatic cervical cancer without reducing quality of life; however, it increases the likelihood of gastrointestinal fistula (14,20). In this case, the patient was too frail to tolerate chemotherapy. However, she developed a severe intestinal fistula 10 days after bevacizumab and 4 days after seed implantation. The patient died 50 days later of a serious, uncontrollable general infection. The cause was considered to be radiological damage from a high cumulative dose, but we could not exclude the anti-angiogenic mechanism of bevacizumab. Further, it is debatable whether the secondary implantation and bevacizumab were overtreatment.
We share our experience and consider this complex case of multiple recurrences of cervical cancer. Although the patient died, multimodality treatment prolonged her survival, alleviated her symptoms, and improved her quality of life. In summary, our experience suggests that PE offers certain benefits in the absence of better options, even in patients with pelvic wall invasion. However, for large tumours where preoperative examination reveals pelvic wall and bladder invasion, appropriate extension of resection, IORT, or neoadjuvant chemotherapy should be considered. Under these conditions, re-irradiation should be considered cautiously, and modalities that will protect the OARs as much as possible are preferable. EBRT is a potential treatment for small and scattered lesions. There is currently no standardised dose; therefore, the appropriate dose should be determined by experienced clinicians. In this case, the first radioactive seed implantation demonstrated its potential, but the cause of the fistula after secondary implantation could not be determined. Non-invasive re-irradiation, such as SBRT, may be a better choice when there is a poor healing incision after re-recurrence.
Acknowledgments
We would like to thank Editage (https://www.editage.cn) for English language editing.
Funding: This work was supported by Jilin University [2013106022]; and Department of Science and Technology of Jilin Province, China (20160101119JC); and Special Project of Medical and Health Professionals of Jilin Province, China (3D5197457429); and Department of Public Health of Jilin Province, China (2015Z009); and The National High Technology Research and Development Program of China (2017YFC0113702); and Jilin Province Financial and Health Project, China (Research on the Differential Expression of Cyclic RNA in Cervical Cancer HeLa Cells and the Mechanism of Radiation Resistance under Radiation Induction); and Jilin Provincial Department of Finance (Consortium of Medical Consortium for Diagnosis and Treatment of Difficult Women’s Tumors and Precision Radiotherapy Training Base Construction Project).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2250/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2250/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Legge F, Chiantera V, Macchia G, et al. Clinical outcome of recurrent locally advanced cervical cancer (LACC) submitted to primary multimodality therapies. Gynecol Oncol 2015;138:83-8. [Crossref] [PubMed]
- Friedlander M, Grogan MU.S. Preventative Services Task Force. Guidelines for the treatment of recurrent and metastatic cervical cancer. Oncologist 2002;7:342-7. [Crossref] [PubMed]
- Berek JS, Howe C, Lagasse LD, et al. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol 2005;99:153-9. [Crossref] [PubMed]
- Foley OW, Rauh-Hain JA, del Carmen MG. The role of intraoperative radiation therapy in the management of recurrent and locally advanced gynecologic cancers. Int J Gynecol Cancer 2013;23:9-15. [Crossref] [PubMed]
- Biete A, Oses G. Intraoperative radiation therapy in uterine cervical cancer: A review. Rep Pract Oncol Radiother 2018;23:589-94. [Crossref] [PubMed]
- Höckel M. Laterally extended endopelvic resection. Novel surgical treatment of locally recurrent cervical carcinoma involving the pelvic side wall. Gynecol Oncol 2003;91:369-77. [PubMed]
- Kim HJ, Chang JS, Koom WS, et al. Radiotherapy is a safe and effective salvage treatment for recurrent cervical cancer. Gynecol Oncol 2018;151:208-14. [Crossref] [PubMed]
- Martínez-Monge R, Cambeiro M, Rodríguez-Ruiz ME, et al. Phase II trial of image-based high-dose-rate interstitial brachytherapy for previously irradiated gynecologic cancer. Brachytherapy 2014;13:219-24. [Crossref] [PubMed]
- Mahantshetty U, Kalyani N, Engineer R, et al. Reirradiation using high-dose-rate brachytherapy in recurrent carcinoma of uterine cervix. Brachytherapy 2014;13:548-53. [Crossref] [PubMed]
- Mabuchi S, Takahashi R, Isohashi F, et al. Reirradiation using high-dose-rate interstitial brachytherapy for locally recurrent cervical cancer: a single institutional experience. Int J Gynecol Cancer 2014;24:141-8. [Crossref] [PubMed]
- Han L, Li C, Wang J, et al. Iodine-125 radioactive seed tissue implantation as a remedy treatment for recurrent cervical cancer. J Cancer Res Ther 2016;12:C176-80. [Crossref] [PubMed]
- Qu A, Jiang P, Sun H, et al. Efficacy and dosimetry analysis of image-guided radioactive 125I seed implantation as salvage treatment for pelvic recurrent cervical cancer after external beam radiotherapy. J Gynecol Oncol 2019;30:e9. [Crossref] [PubMed]
- Tong L, Liu P, Huo B, et al. CT-guided 125I interstitial brachytherapy for pelvic recurrent cervical carcinoma after radiotherapy. Onco Targets Ther 2017;10:4081-8. [Crossref] [PubMed]
- Penson RT, Huang HQ, Wenzel LB, et al. Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240). Lancet Oncol 2015;16:301-11. [Crossref] [PubMed]
- Liu ZS, Guo J, Zhao YZ, et al. Computed Tomography-Guided Interstitial Brachytherapy for Locally Advanced Cervical Cancer: Introduction of the Technique and a Comparison of Dosimetry With Conventional Intracavitary Brachytherapy. Int J Gynecol Cancer 2017;27:768-75. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Mabuchi S, Matsumoto Y, Komura N, et al. The efficacy of surgical treatment of recurrent or persistent cervical cancer that develops in a previously irradiated field: a monoinstitutional experience. Int J Clin Oncol 2017;22:927-36. [Crossref] [PubMed]
- Chao X, Song X, Wu H, et al. Selection of Treatment Regimens for Recurrent Cervical Cancer. Front Oncol 2021;11:618485. [Crossref] [PubMed]
- Park HJ, Chang AR, Seo Y, et al. Stereotactic Body Radiotherapy for Recurrent or Oligometastatic Uterine Cervix Cancer: A Cooperative Study of the Korean Radiation Oncology Group (KROG 14-11). Anticancer Res 2015;35:5103-10. [PubMed]
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654-63. [Crossref] [PubMed]



