
A systematic review and meta-analysis on the risk factors of acute myeloid leukemia
Introduction
Leukemia is a heterogeneous, malignant, and clonal disease. Mutations in hematopoietic stem cells or progenitor cells may be the main cause of the disease. Its main clinical manifestations are abnormal blood cells, bone marrow and uncontrolled infiltration and growth of other hematopoietic tissues, which inhibit normal hematopoietic tissues and further produce corresponding clinical manifestations (1). Clinical studies have shown that about 34% of leukemia patients who are identified each year have acute myeloid leukemia (AML), 28% have chronic lymphocytic leukemia (CLL), 13% have chronic myeloid leukemia (CML), and 11% have acute lymphocytic leukemia (ALL) (2). From the above findings, it can be seen that AML is one of the main types of leukemia (3). Clinical studies have shown that ALL occurs mostly in children between the ages of 2 and 10, and the disease is the second leading cause of death in patients under 15 years of age (4). In contrast, the incidence of AML gradually increases with age, the median age of onset is 60 years old.
There is no consensus on the cause of leukemia. At present, 3 factors are considered to be related to the onset of leukemia, namely ionizing radiation, benzene, and alkylating agents (5-7). However, the currently known risk factors can only explain the pathogenesis of a small number of patients, while the pathogenesis of most of these diseases remain to be elucidated. Recent studies have shown that smoking, electromagnetic fields, hair dye, organic solvents, and viral infections may be risk factors for leukemia (8-11). Since the beginning of the century, there has been gradual development of artificial electromagnetic fields, especially low-frequency electromagnetic fields such as power plants, radios, radars, televisions, computers, mobile phones, microwave ovens, and countless large-scale equipment used in medical treatment and factories. Technological advancements such as these have raised concerns about the health effects of unprecedented levels of electromagnetic field exposure. At present, related studies have reported the relationship between high-voltage lines in the environment and childhood blood cancer (12). Some scientists have pointed out that exposure to the electromagnetic field environment generated by the high-voltage power line system is related to several types of cancer. For example, cancers such as leukemia, breast cancer, and melanoma, among others, can cause fertility disorders, birth defects, neurological disorders, and Alzheimer’s disease (13). However, a limited number of studies have demonstrated that exposure to electromagnetic fields in residential or workplace environments can cause adult leukemia (14).
At present, the etiology of acute leukemia is mostly related to some clinical studies, these studies have not reached a unified conclusion, and there are few articles related to systematic summary analysis. This study analyzed a total of 10 articles, reviewed the results of domestic and foreign case comparison studies, and carried out screening and meta-analysis to explore the risk factors of AML, so as to provide a reference and basis for better prevention and treatment of the disease in clinical practice. We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-27/rc).
Methods
Strategies for article retrieval
The literature was searched from Medline, Excerpta Medica Database (EMBASE), EBSCO, OVID, Chinese Biology Medicine Disc (CBMDISC), Web of Science, and Wanfang by taking “acute myeloid leukemia”, “AML”, “exposure factor”, and “risk factors” as key words. The key words were combined with “or” and “and” for joint search. It should search for clinical studies related to the risk of AML that have been published in the period from the establishment of the database to September 2021. All search keywords were freely combined and input into each database to search the target literature. There were no language restrictions when searching.
Inclusion and exclusion criteria
Inclusion criteria for this article were given as follows: the study was a case-control study; the cases were clearly diagnosed as AML patients, and the specific type was not limited; the research content was the risk factors of AML; baseline data of cases and controls were comparable; and the article detailed the gender, age, sample size, risk factors, and data of AML patients.
Exclusion criteria were as follows: articles which were individual case reports, literature reviews, and reports without primary data; articles reporting only deaths and accidental exposures; literature on animal experiments; repeated studies; and articles which were rated on the Newcastle-Ottawa Scale (NOS) low-quality research.
Article screening
The article screening and data extraction were independently conducted, and then cross-checking was performed. If there were different opinions, experts were consulted to decide the data selection.
Information extraction
Two researchers independently read the included articles to check whether the article was a case-control or cohort study and whether the data was complete. According to the requirements of meta-analysis, all relevant articles that met the inclusion criteria were screened out, and the quality of the articles was evaluated to exclude duplicate reports, those of poor quality, and too little confidence. The data was extracted according to the established tables, and a database was established to check the data. If the research report was incomplete, the author was contacted for verification, and the articles that were confirmed to be unavailable were excluded from this study. If the two researchers disagreed, a discussion would be held with a third party to solve the problem. After the full text was obtained, data was extracted. If there were repeated reports, the most recent research was selected. The data to be extracted for this study included basic information (document title, first author, publication year, author information, and source), basic characteristics of subjects (gender, age, research sample size, and baseline comparability), research methods, research plan design, intervention measures of the experimental group and control group, outcome evaluation indicators, and outcome data.
Data extraction
The forest map also makes clear the results of individual studies, combining those studies with corresponding confidence intervals (CIs). If there is no overlap between the trust intervals of the results of each study, it indicates statistical heterogeneity between the studies. Further subgroup analysis is required to combine stochastic and fixed models with acceptable inhomogeneity. It can be divided into subgroups according to different designs. Then, the influence size of each subgroup can be ignored when the heterogeneity between studies can’t be ignored and different properties can’t be dealt with for the heterogeneity. Select the combined statistical model of the statistical model. Sensitivity analysis: sensitivity analysis of study results by investigating whether individual studies affect the overall results of combinations. Each study included in the study was removed at a time, and combined with the results of the remaining studies, the combined results of each study were compared with their own results to confirm whether the results were the same. In general, this study is expected to have an impact on the comprehensive study in the following two situations. First, if a study is deleted, the presumed size of the combined effect is 95% greater than the size of the combined effect, and the results will be significantly different when a study is deleted. If there is little difference in the results of one study affecting the whole, it indicates the sensitivity of the combined results and the results obtained are unstable. On the contrary, the results show that the sensitivity is stable and the conclusion is correct.
Quality evaluation
The NOS was used to evaluate the quality of the literature. NOS was suitable for the quality evaluation of case-control studies and cohort studies, mainly including three parts and a total of eight aspects. The NOS scale included 4 points for study population selection; 2 points for comparability between groups; 3 points for appropriate measurement of exposure factors, or 3 points for appropriate outcome measures. The full score was 9 points, and the high-quality literature was above 6 points.
Statistical analysis
RevMan5.3 software was used for meta-analysis of the included literature. The calculation method used risk ratio (RR) or odds ratio (OR) as the effect size, and 95% CI was adopted to express the result. A heterogeneity test was performed on the included articles, taking α=0.1 as the test level. If there was no heterogeneity among the included articles (P>0.1; I2<50%), the fixed effects model (FEM) was selected for meta-analysis. Otherwise, the random effects model (REM) was selected for meta-analysis, and a subgroup analysis was performed on the included data. P<0.05 indicated that the difference was statistically significant. When a single risk factor analysis included more than 10 articles, a funnel plot was used to analyze the publication bias of each risk factor.
Results
Literature search, basic data, and quality assessment
A total of 3,366 related articles were retrieved in this study. According to the requirements, 1,389 articles were initially eliminated, and 1,451 articles that obviously did not meet the inclusion criteria were eliminated after the titles and abstracts were read. Subsequently, 513 articles were eliminated through simple reading of the full texts, and after careful reading of the articles, 3 articles with unclear grouping and unclear outcome indicators were excluded. Finally, 10 articles (15-24) that met the inclusion criteria were included. The quality of the literature was evaluated by NOS, 2 papers were scored 6 points, 3 papers were scored 7 points, and 5 papers were scored 8 points. Therefore, the quality of the included literature was high. The details are shown in Figure 1 and Table 1.
Table 1
| First author | Number of cases | Controls | Year of publication | Country | Region | NOS score |
|---|---|---|---|---|---|---|
| Wen WQ | 302 | 558 | 1998 | America/Canada | America | 6 |
| Perrillat F | 279 | 285 | 2001 | France | Europe | 8 |
| Rudant J | 773 | 1,681 | 2007 | France | Europe | 8 |
| Ripert M | 472 | 567 | 2007 | France | Europe | 7 |
| Gentile G | 431 | 862 | 1996 | Italy | Europe | 6 |
| Anderson LA | 61,464 | 122,531 | 2008 | America | America | 7 |
| Kang J | 3,932 | 15,562 | 2011 | North Korea | Asia | 8 |
| Gao Z | 958 | 785 | 2018 | China | Asia | 8 |
| Ding G | 176 | 180 | 2012 | China | Asia | 7 |
| Shih TY | 58 | 232 | 2014 | China | Asia | 8 |
NOS, Newcastle-Ottawa Scale.
Relationship between family history of tumor and AML
Meta-analysis was performed on 4 AML-related articles through article screening. During the analysis, “with a family history of tumors” was the exposure, while the control group studied cases with “no family history of tumors”. The definitions of exposure/non-exposure in all articles were similar. The heterogeneity analysis results showed that I2=94% and P<0.00001. According to the early article, I2=94% was greater than 50%, so the REM was used for analysis. The combined effect size result was RR (95% CI): 0.98 (0.57, 1.69); Z=0.08; P=0.94. The analysis results showed that there was a difference between the experimental group and the control group in terms of family history of tumor, but the difference was not significant (P>0.05). This suggests that family history of tumor may be one of the risk factors for AML. The forest plot between family history of tumor and AML is shown in Figure 2.

The relationship between hepatitis B virus (HBV) infection rate and leukemia
Meta-analysis was performed on 3 articles of AML patients and the general population obtained through article screening. In the analysis process, “with HBV” was the exposure, while the control group studied cases with “no HBV”. The definitions of exposure/non-exposure in the studies were similar. The heterogeneity analysis results showed that I2=98% and P<0.00001, so the REM was used for analysis. The combined effect size result was OR (95% CI): 1.34 (0.57, 3.13); Z=0.68; P=0.50. The analysis results showed that there was a difference in the prevalence of HBV between the experimental group and the control group, but the difference was not significant (P>0.05). This suggests that HBV infection may be one of the risk factors for the occurrence of AML. The forest plot between HBV infection rate and AML is shown in Figure 3.

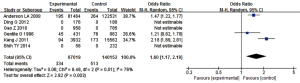
The relationship between hepatitis C virus (HCV) infection rate and AML
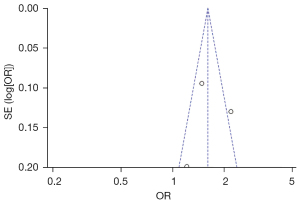
Meta-analysis was performed on 3 articles of AML patients and the general population obtained through article screening. During the analysis, “with HCV” was the exposure, while the control group studied cases with “no HCV”. The definitions of exposure/non-exposure in all articles were similar. The heterogeneity analysis results showed that I2=76% and P=0.01. As I2=76% was greater than 50%, the REM was used for analysis. The combined effect size result was OR (95% CI): 1.60 (1.17, 2.19); Z=2.92; P=0.003. The analysis results showed that there was a difference between the experimental group and the control group in the HCV infection rate, and the difference was significant (P<0.05). This suggests that HCV infection may be one of the risk factors for the occurrence of AML. The forest plot between HCV infection rate and AML is shown in Figure 4. Figure 5 is an inverted funnel plot of the relationship analysis between the 2. As shown in Figure 5, the funnel plot is basically symmetrical, and most of the data corresponded to points within the 95% CI, suggesting that the quality of the included articles was high and the risk of deviation was low.
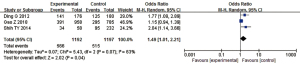
The relationship between environmental exposure and AML
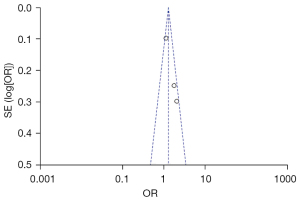
Meta-analysis was performed on 3 articles of AML patients and the general population obtained through article screening. In the analysis process, “with a history of home decoration, X-ray inspection, and exposure to pesticides” were the exposures, while the control group studied cases of “no history of home decoration, no X-ray inspection, and no exposure to pesticides”. The definitions of exposure/non-exposure in all articles were similar. The heterogeneity analysis results showed that I2=63% and P=0.07, I2=63%, which was greater than 50%, so the REM was used for analysis. The combined effect size result was OR (95% CI): 1.49 (1.01, 2.21); Z=2.02; P=0.04. The analysis results showed that the experimental group and the control group were significantly different in terms of history of home decoration, X-ray inspection, and exposure to pesticides (P<0.05). This suggests that home decoration history, X-ray inspection, and pesticide exposure may be risk factors for AML. The forest plot between home decoration history, X-ray inspection, pesticide exposure, and AML is shown in Figure 6. Figure 7 shows a funnel plot of the relationship analysis between them. As shown in Figure 7, the funnel plot is basically symmetrical, and most of the data correspond to points within the 95% CI, suggesting that that the quality of the included articles was high and the risk of deviation was low.
Discussion
Leukemia threatens human life and health, and its incidence rate is increasing year by year worldwide. Epidemiological data show that the incidence of leukemia is highest in Mexico City and Latin America, with 5.80 cases per 100,000. The annual incidence rate of leukemia in China from 1986–1988 was 2.76 cases per 100,000 (25). Statistics from 2003 to 2007 showed that the incidence of leukemia in China was 5.17 cases per 100,000, a significant increase compared to the survey results in 1986–1988, with a mortality rate of 3.94 cases per 100,000. The morbidity population is mainly those under the age of 35, including the 2 main morbidity groups of adults and children (26). Leukemias are generally classified into AML, CLL, CML, and ALL. Among them, AML and CLL are the most common and frequent. Clinical data show that about 34% of leukemia cases which develop each year are AML, 28% are CLL, 13% are CML, and 11% are ALL (27). Adult leukemia in China accounts for 10% of malignant tumors and adult AML accounts for 60–70% of newly diagnosed adult leukemia and its incidence is increasing year by year. In addition, the morbidity rate also gradually increases with age, and the mortality rate of adult AML ranks sixth among malignant tumors (28). The incidence of AML in children is lower than that in adults, but it is still as high as 25% (29).
The pathogenesis of AML is currently unclear. Although many studies have revealed that viruses may be the main factor. In addition, some cytotoxic substances (including alkylating agents and topoisomerase inhibitors, ionizing radiation, benzene, and other risk factors) can also cause chromosome breakage and susceptibility. This can make the position of the oncogene move and become activated and cell mutation and immune function to decline, which is conducive to the occurrence of leukemia (30,31). However, the medical community has not yet clarified whether the causes of hair dyeing, smoking, obesity, green tea, radiation, and other lifestyle factors have a certain impact on the occurrence of leukemia. With the continuous modernization of lifestyles, these possible risk factors for the onset of leukemia have become more and more closely related to people’s lives (32). In addition, China is a major hepatitis country, and one-tenth of population is a carrier of HBV. At the same time, whether family history of tumors is a risk factor for predicting the onset of AML is also a controversial issue (33). For children, in addition to the above factors, they will also be affected by maternal factors related to pregnancy. For example, relevant studies show that maternal hair perming, drinking and smoking during pregnancy will increase the risk of leukemia in children (34). Therefore, it is necessary to explore the causes of AML. In this study, the etiology of AML was investigated. A total of 10 articles were included. Meta-analysis of risk factors for AML was carried out from the perspectives of family tumor history, HBV, HCV, and environmental exposure. The analysis results showed that family tumor history, HBV, HCV, and environmental exposure were all closely related to the onset of AML and were risk factors for AML.
Conclusions
This study included 10 articles and explored the influence of factors such as family tumor history, HBV infection rate, HCV infection rate, and environmental exposure rate on the incidence of AML. The results showed that HCV infection and environmental exposure were risk factors for the onset of AML. Whether family tumor history and HBV infection are risk factors for the onset of AML should be studied in further clinical trials to verify the results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-27/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pelcovits A, Niroula R. Acute Myeloid Leukemia: A Review. R I Med J (2013) 2020;103:38-40. [PubMed]
- Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev 2017;31:63-76. [Crossref] [PubMed]
- Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol 2018;93:1267-91. [Crossref] [PubMed]
- Medinger M, Heim D, Halter JP, et al. Diagnosis and Therapy of Acute Myeloid Leukemia. Ther Umsch 2019;76:481-6. [Crossref] [PubMed]
- Elgarten CW, Aplenc R. Pediatric acute myeloid leukemia: updates on biology, risk stratification, and therapy. Curr Opin Pediatr 2020;32:57-66. [Crossref] [PubMed]
- Shanmuganathan N, Hiwase DK, Ross DM. Treatment of chronic myeloid leukemia: assessing risk, monitoring response, and optimizing outcome. Leuk Lymphoma 2017;58:2799-810. [Crossref] [PubMed]
- Hou HA, Tien HF. Genomic landscape in acute myeloid leukemia and its implications in risk classification and targeted therapies. J Biomed Sci 2020;27:81. [Crossref] [PubMed]
- Song X, Peng Y, Wang X, et al. Incidence, Survival, and Risk Factors for Adults with Acute Myeloid Leukemia Not Otherwise Specified and Acute Myeloid Leukemia with Recurrent Genetic Abnormalities: Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database, 2001-2013. Acta Haematol 2018;139:115-27. [Crossref] [PubMed]
- Berlivet J, Hémon D, Cléro É, et al. Residential exposure to natural background radiation at birth and risk of childhood acute leukemia in France, 1990-2009. J Environ Radioact 2021;233:106613. [Crossref] [PubMed]
- Couto AC, Ferreira JD, Rosa AC, et al. Pregnancy, maternal exposure to hair dyes and hair straightening cosmetics, and early age leukemia. Chem Biol Interact 2013;205:46-52. [Crossref] [PubMed]
- Metayer C, Scelo G, Kang AY, et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ Res 2016;151:174-83. [Crossref] [PubMed]
- Silva-Junior AL, Alves FS, Kerr MWA, et al. Acute lymphoid and myeloid leukemia in a Brazilian Amazon population: Epidemiology and predictors of comorbidity and deaths. PLoS One 2019;14:e0221518. [Crossref] [PubMed]
- Errico Provenzano A, Amatori S, Nasoni MG, et al. Effects of Fifty-Hertz Electromagnetic Fields on Granulocytic Differentiation of ATRA-Treated Acute Promyelocytic Leukemia NB4 Cells. Cell Physiol Biochem 2018;46:389-400. [Crossref] [PubMed]
- Storch K, Dickreuter E, Artati A, et al. BEMER Electromagnetic Field Therapy Reduces Cancer Cell Radioresistance by Enhanced ROS Formation and Induced DNA Damage. PLoS One 2016;11:e0167931. [Crossref] [PubMed]
- Wen WQ, Shu XO, Sellers T, et al. Family history of cancer and autoimmune disease and risk of leukemia in infancy: a report from the Children's Cancer Group (United States and Canada). Cancer Causes Control 1998;9:161-71. [Crossref] [PubMed]
- Perrillat F, Clavel J, Jaussent I, et al. Family cancer history and risk of childhood acute leukemia (France). Cancer Causes Control 2001;12:935-41. [Crossref] [PubMed]
- Rudant J, Menegaux F, Leverger G, et al. Family history of cancer in children with acute leukemia, Hodgkin's lymphoma or non-Hodgkin's lymphoma: the ESCALE study (SFCE). Int J Cancer 2007;121:119-26. [Crossref] [PubMed]
- Ripert M, Menegaux F, Perel Y, et al. Familial history of cancer and childhood acute leukemia: a French population-based case-control study. Eur J Cancer Prev 2007;16:466-70. [Crossref] [PubMed]
- Kang J, Cho JH, Suh CW, et al. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol 2011;90:159-64. [Crossref] [PubMed]
- Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev 2008;17:3069-75. [Crossref] [PubMed]
- Gentile G, Mele A, Monarco B, et al. Hepatitis B and C viruses, human T-cell lymphotropic virus types I and II, and leukemias: a case-control study. The Italian Leukemia Study Group. Cancer Epidemiol Biomarkers Prev 1996;5:227-30. [PubMed]
- Gao Z, Wang R, Qin ZX, et al. Protective effect of breastfeeding against childhood leukemia in Zhejiang Province, P. R. China: a retrospective case-control study. Libyan J Med 2018;13:1508273. [PubMed]
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol 2012;46:13480-7. [Crossref] [PubMed]
- Shih TY, Wu J, Muo CS, et al. Association between leukaemia and X-ray in children: a nationwide study. J Paediatr Child Health 2014;50:615-8. [Crossref] [PubMed]
- Tallman MS, Wang ES, Altman JK, et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:721-49. [Crossref] [PubMed]
- Wei AH, Strickland SA Jr, Hou JZ, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol 2019;37:1277-84. [Crossref] [PubMed]
- Burchert A, Bug G, Fritz LV, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol 2020;38:2993-3002. [Crossref] [PubMed]
- Herold T, Rothenberg-Thurley M, Grunwald VV, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2020;34:3161-72. [Crossref] [PubMed]
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv 2020;4:3528-49. [Crossref] [PubMed]
- Ye X, Chen D, Zheng Y, et al. The incidence, risk factors, and survival of acute myeloid leukemia secondary to myelodysplastic syndrome: A population-based study. Hematol Oncol 2019;37:438-46. [Crossref] [PubMed]
- Young AL, Tong RS, Birmann BM, et al. Clonal hematopoiesis and risk of acute myeloid leukemia. Haematologica 2019;104:2410-7. [Crossref] [PubMed]
- Atteya A, Ahmad A, Daghstani D, et al. Evaluation of Hepatitis B Reactivation Among Patients With Chronic Myeloid Leukemia Treated With Tyrosine Kinase Inhibitors. Cancer Control 2020;27:1073274820976594. [Crossref] [PubMed]
- Logan C, Koura D, Taplitz R. Updates in infection risk and management in acute leukemia. Hematology Am Soc Hematol Educ Program 2020;2020:135-9. [Crossref] [PubMed]
- Timms JA, Relton CL, Sharp GC, et al. Exploring a potential mechanistic role of DNA methylation in the relationship between in utero and post-natal environmental exposures and risk of childhood acute lymphoblastic leukaemia. Int J Cancer 2019;145:2933-43. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)



