
Pathological complete response to radical surgery after receiving durvalumab plus neoadjuvant chemotherapy for 1 limited-stage small cell lung cancer patient: a case report
Introduction
In recent years, lung cancer has become the most common cancer around the world (1). It is also the leading cause of cancer-related deaths worldwide, and accounts for 18.4% of all cancer-related deaths (1). Small cell lung cancer (SCLC) accounts for about 15% of lung cancer cases. It is characterized by rapid growth and extensive early metastasis (1). Under the classification of The Veterans’ Administration Lung Study Group, SCLC include limited-stage SCLC (LS-SCLC) and extensive-stage SCLC (ES-SCLC) (2). LS-SCLC refers to cases that are confined to the ipsilateral hemithorax and local lymph nodes, and can be safely surrounded by a single radiation field. The cases that initially cannot receive radiotherapy are classified as ES-SCLC (3).
Surgery plus adjuvant chemotherapy is the standard treatment for patients with limited T1–2N0 SCLC. Studies have shown that patients with lymph node negative SCLC treated with surgery plus adjuvant chemotherapy could receive longer survival compared with surgery alone or concurrent chemoradiotherapy (4,5). Etoposide + cisplatin (6) or etoposide + carboplatin (7) are the first choice for adjuvant chemotherapy. However, for LS-SCLC beyond T1–2N0M0, additional chest radiotherapy is better than chemotherapy alone (8), and concurrent chemoradiotherapy is better than sequential chemoradiotherapy (9). Currently, the optimal dose and scheme of chest radiotherapy are unknown. The majority of patients respond to initial therapy, but then they will experience disease relapse. The overall survival (OS) rate of LS-SCLC patients is only 30–35% (10).
Durvalumab is a programmed death-ligand 1 (PD-L1) blocking antibody that enables T cells to recognize and kill tumor cells (11). In the CASPIAN study, the first-line treatment of durvalumab plus platinum-etoposide significantly improved the OS of patients with ES-SCLC (12). However, for LS-SCLC, there have been no breakthroughs in immunotherapy. The ADRIATIC trial (ClinicalTrials.gov identifier, NCT03703297), an ongoing global randomized clinical trial (RCT), investigates the efficacy and safety of durvalumab with or without tremelimumab for patients with LS-SCLC after finishing concurrent chemoradiotherapy. We sincerely hope that the data of ADRIATIC trial will change the treatment landscape of LS-SCLC. In LS-SCLC, surgical treatment is only applicable to T1–2N0 patients. Radiotherapy and chemotherapy are still of great significance in the treatment of patients with LS-SCLC. The initial response rate is high, but relapsed and refractory patients are still difficult to treat. Many clinical trials have examined the use of immune checkpoint inhibitors plus neoadjuvant chemotherapy in treating resectable NSCLC, and found that this treatment achieves good clinical outcomes (13,14). Thus, we hypothesized that durvalumab plus neoadjuvant chemotherapy would also be a good treatment option for LS-SCLC.
In this article, we report for the first time a case of LS-SCLC who showed a pathological complete response after receiving durvalumab plus neoadjuvant chemotherapy and radical surgery. It’s a very valuable case and we hope this case can provide some treatment experience for LS-SCLC in the future. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-729/rc).
Case presentation
On April 21, 2020, a 75-year-old male was hospitalized mainly for cough, expectoration, and bloody sputum. The patient had smoked about 25 cigarettes per day for 40 years, but had quit smoking 20 days ago. He had also drunk alcohol for 20 years, 150 ml each time, but had stopped drinking 3 years ago. No other special personal or family history was reported. The patient had a performance-status score of 2 points. The tumor markers showed that the pro-gastrin-releasing peptide (ProGRP) level was 677.50 pg/mL and the neuron-specific enolase (NSE) level was 21.61 ng/mL, both of which were higher than normal values. The bronchoscopy showed local uplift and hyperplasia of bronchial mucosa in the basal segment of the lower lobe of left lung. The pathology and immunohistochemistry of the bite examination suggested small cell carcinoma. Computed tomography (CT) imaging showed masses in the left lower hilum and left lower lobe of the lung, micronodules in the left lower lobe, interstitial changes in the lower lobe of both lungs, emphysema, a small cyst in the left lobe of the liver, and multiple cysts in both kidneys (see Figure 1). Skull CT and bone imaging showed no abnormalities. The patient was diagnosed with LS small cell carcinoma of the left lung (tumor, node metastasis stage cT3N0M0 IIB).
According to the Chinese Society of Clinical Oncology guidelines, the role of surgery in stage IIB–IIIA SCLC is still controversial, and concurrent chemoradiotherapy is recommended. Based on the findings of the CASPIAN study, we decided to administer durvalumab plus neoadjuvant chemotherapy. On April 22, 2020 and May 19, 2020, the patient received etoposide (0.5 g) plus lobaplatin (50 mg) and durvalumab (500 mg) for 2 cycles. The process was favorable and no adverse events were observed. After 2 cycles, the ProGRP and NSE levels decreased to 62.23 pg/mL and 12.28 ng/mL, respectively, and both returned to the normal values. The CT re-examination showed post-chemotherapy changes in the left lower lobe, a slightly larger pulmonary hilum than normal, and a smaller range than before (see Figure 2). At baseline, the diameter of the tumor in the left lower hilum of the lung was 4.3 cm, and the diameter of the tumor in the left lower lobe was 3.6 cm. After two cycles of neoadjuvant therapy, we can see no clear mass on the CT of the lung (see Figure 2). The assessment of the tumor via Resist1.1 by videography indicated a complete response (15). On June 28, 2020, a thoracoscopic resection of the left lower lobe of the lung was performed under general anesthesia. The operation was successful. The patient’s postoperative recovery was good, and the patient was discharged smoothly.
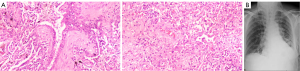
The postoperative pathology results showed that the lung tissue was 15 cm × 11 cm × 4 cm and the bronchial mucosa was smooth, and no obvious tumor was observed in the multi section incision (see Figure 3A). The lung tissue of the tumor bed showed chronic inflammation and carbon dust deposition, and no residual cancer was found. The results were as follows: bronchial stump (−); lymph nodes: 0/1 in group 5, 0/2 in group 7, 0/2 in group 9, 0/3 in group 10, and 0/11 in group 11; and stage: ypT0N0M0. A chest X-ray examination was performed after surgery (see Figure 3B). Postoperative changes in the cancer in the left lung were observed. After the surgery, the patient received etoposide (0.5 g) plus lobaplatin (50 mg) and durvalumab (500 mg) for 3 cycles. Subsequently, the patient received regular reexamination and follow-up. Now, the patient is still alive and there is no sign of tumor recurrence. The timeline of the case is shown in Figure 4.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Here, we reported a case of a 75-year-old male patient with LS-SCLC of the left lung (tumor, node metastasis stage cT3N0M0 IIB) who received 2 cycles durvalumab plus neoadjuvant chemotherapy. The neoadjuvant therapy was favorable and no adverse events were observed. Then a thoracoscopic resection of the left lower lobe of the lung was performed under general anesthesia. The operation was successful, and the patient’s postoperative recovery was good. Fortunately, The postoperative pathology results showed pathological complete response. After the surgery, the patient received 3 cycles of adjuvant therapy. Now, the patient is still alive and there is no sign of tumor recurrence.
Cytotoxic T cells are key for the immune system to kill tumors, and the PD-L1/programmed cell death protein 1 (PD-1) pathway is the main pathway for immune escape. PD-1/PD-L1 inhibits T cell function through multiple signaling pathways, enabling PD-(L)1 inhibitors to play an anti-tumor role by promoting immune normalization (14). Durvalumab is an anti-programmed cell death receptor-1 antibody that blocks the PD-L1 from binding to the PD-1 and CD80, thus helping to restore the endogenous anti-tumor T cell response.
In the CASPIAN study, the researchers evaluated the efficacy of durvalumab combined with or without tremelimumab + etoposide plus cisplatin or carboplatin in patients who had been newly diagnosed with ES-SCLC. In total, 268 patients were randomly assigned to the durvalumab combined with platinum + etoposide group, and 269 patients were randomly assigned to the platinum + etoposide group. Durvalumab combined with platinum + etoposide significantly improved patients’ OS (12). These findings suggested that the combination of durvalumab and platinum + etoposide significantly improved the OS of patients with ES-SCLC. Based on the results of this study, durvalumab in combination with etoposide and either carboplatin or cisplatin as a first-line treatment of ES-SCLC has been approved by Food and Drug Administration (16). Currently, there is very little evidence about the effect of immune checkpoint inhibitors on LS-SCLC.
For patients with stage I–IIA LS-SCLC, research has shown that the 5-year survival rates of the surgical group and the non-surgical group are 27–73% and 4–44%, respectively, which suggests that patients can benefit from surgery (17). In a propensity-matching analysis based on the National Cancer Database, Yang et al. (4) found that surgical treatment compared with concurrent chemoradiation significantly improved the 5-year survival rate of patients (47.6% vs. 29.8%; P<0.01). In terms of surgical methods, a number of retrospective studies and meta-analyses (4,18) have shown that the survival of the lobectomy group was better than that of the wedge-resection group.
However, for patients with stage IIB–IIIA LS-SCLC, the role of surgery is still controversial (17). Some retrospective studies (17) have found positive results, but the median survival time obtained in these studies (17) ranges from 17–31.7 months, which does not represent a breakthrough compared to the 25 months in the CONVERT study (19) with concurrent chemoradiotherapy. Thus, the effectiveness and suitability of surgery for stage IIB–IIIA SCLC remains unclear.
Radical surgery is only recommended for LS-SCLC patients with T1–2N0M0 cancer staging, and <5% of patients with SCLC meet this criteria at the time of their initial diagnosis of SCLC (20). Most LS-SCLC patients are beyond T1–2N0, and should receive concurrent chemoradiotherapy. In the first-line treatment, the effective rate is about 80%, but the majority of patients will relapse in 6 months after finishing the initial treatment (21). Here is a study to compare the efficacy of surgery with concurrent chemoradiotherapy in the patients diagnosed with LS-SCLC (22), 102 patients received concurrent chemoradiotherapy were involved into the CCRT group (the patients who received concurrent chemoradiotherapy), and the CCRT group was selected as control group. Referring to the order of adjuvant therapy, S group contain SA group and NS group. There are 30 patients in SA group, and these patients received adjuvant chemotherapy and radiotherapy. Different from SA group, there were 20 cases in NS group, and the patients received neoadjuvant chemotherapy. The results indicated that both the median progression-free survival (PFS) and the median OS of the S group were notably longer than the CCRT group (P<0.0001). In subgroup survival analysis, we can saw that there were no significant difference between NS group and SA group. This study indicated that, for LS-SCLC patients, radical surgery combined with perioperative chemotherapy and adjuvant radiotherapy may be a better choice.
It is clear that preoperative neoadjuvant chemotherapy has better survival benefits than surgery alone or concurrent chemoradiotherapy alone, but there is still a long way to go to optimize the benefit of long-term survival. Several previous studies have shown that immunotherapy has great potential in the neoadjuvant therapy of NSCLC, and research has shown that 20–85% of patients achieve major pathological remission, which is better than the findings of previous neoadjuvant chemotherapy studies (23,24). However, research on immunotherapy in neoadjuvant therapy in LS-SCLC are limited. The use of targeted drugs in the treatment of NSCLC has been shown to significantly improve the clinical prognosis and quality of life of patients (25). However, for LS-SCLC, the use of targeted drugs is still in the research stage, and there are no clinical trial findings confirming their efficacy (26).
In this case, we tried a new method in the treatment of LS-SCLC, and found that durvalumab plus neoadjuvant chemotherapy had promising anti-tumor efficacy. The strengths of the treatment are the patient received pathological complete response and there were no adverse event. Furthermore, there are still some limitations of this case. Firstly, further research needs to be conducted to determine whether the pathological complete response can turn into long-term survival. Secondly, the post-surgery treatment of this patient still needs to be explored. Thirdly, we should conduct a randomized control trial of large samples to verify the efficacy and safety of this treatment. Inconclusion, this case provides a new treatment option for patients with LS-SCLC, and we can see the effect of immunotherapy plus neoadjuvant chemotherapy in treating SCLC, which should be further explored in the future. Further, more clinical trials are needed to provide stronger evidence to guide the clinical practice of LS-SCLC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-729/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-729/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Kalemkerian GP. Staging and imaging of small cell lung cancer. Cancer Imaging 2012;11:253-8. [Crossref] [PubMed]
- Nosaki K, Seto T. The Role of Radiotherapy in the Treatment of Small-Cell Lung Cancer. Curr Treat Options Oncol 2015;16:56. [Crossref] [PubMed]
- Yang CJ, Chan DY, Shah SA, et al. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann Surg 2018;268:1105-12. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832-8. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054-60. [Crossref] [PubMed]
- Higgins KA, Gorgens S, Sudmeier LJ, et al. Recent developments in limited stage small cell lung cancer. Transl Lung Cancer Res 2019;8:S147-52. [Crossref] [PubMed]
- Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res 2015;3:1052-62. [Crossref] [PubMed]
- Paz-Ares L, Chen Y, Reinmuth N, et al. PL02. 11 Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. J Thorac Oncol 2019;14:S7-8. [Crossref]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Ulas EB, Dickhoff C, Schneiders FL, et al. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open 2021;6:100244. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- FDA approves durvalumab for extensive-stage small cell lung cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-extensive-stage-small-cell-lung-cancer
- Martucci N, Morabito A, La Rocca A, et al. Surgery in Small-Cell Lung Cancer. Cancers (Basel) 2021;13:390. [Crossref] [PubMed]
- Liu T, Chen Z, Dang J, et al. The role of surgery in stage I to III small cell lung cancer: A systematic review and meta-analysis. PLoS One 2018;13:e0210001. [Crossref] [PubMed]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. [Crossref] [PubMed]
- Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014;9:1140-5. [Crossref] [PubMed]
- Zarogoulidis K, Ziogas E, Papagiannis A, et al. Interferon alpha-2a and combined chemotherapy as first line treatment in SCLC patients: a randomized trial. Lung Cancer 1996;15:197-205. [Crossref] [PubMed]
- Zhong L, Suo J, Wang Y, et al. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J Surg Oncol 2020;18:27. [Crossref] [PubMed]
- Chen XY, Yang F. Progress of immune checkpoint inhibitors in neoadjuvant therapy of non-small cell lung cancer. Zhonghua Wai Ke Za Zhi 2019;57:872-7. [PubMed]
- McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis 2014;6:S224-7. [PubMed]
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 2021;27:1345-56. [Crossref] [PubMed]
- Zhang H, Liu Y. Advances in study on the therapy for limited-stage small cell lung cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2013;38:857-62. [PubMed]
(English Language Editor: L. Huleatt)





