
Gene profiling of metastatic small intestinal squamous cell carcinoma after lung squamous cell carcinoma surgery: a case report
Introduction
At present, about half of all lung cancer patients have distant metastasis at diagnosis, with the common metastatic sites being the lung, liver, bone, brain, and adrenal gland. Surprisingly, approximately 4.6–14% of lung cancer patients have gastrointestinal metastasis at autopsy, only 0.3–1.7% of lung cancer patients were found with gastrointestinal metastasis due to abdominal symptoms such as obstruction, perforation, and bleeding (1-3). Sometimes persistent fecal occult blood suggests gastrointestinal metastasis of lung cancer (4). Small intestine and large intestine account for 60% and 25% of gastrointestinal metastases of lung cancer. However, most previous reports mainly discussed the diagnosis and treatment of the disease, but none of the studies looked at the gene profiles of small intestinal cancer and lung cancer to gain insight into the characteristics of the disease. We encountered a case of jejunal squamous cell carcinoma who had lung squamous cell carcinoma surgery, we performed next generation sequencing (NGS) testing and found some interesting genetic mutations. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-481/rc).
Case presentation
Our patient is a 65-year-old male with a history of smoking 15 cigarettes a day, his father died of colon cancer. On July 25, 2019, positron emission tomography-CT (PET-CT) showed left lung malignant tumor with left hilar lymph node metastasis, and no abnormal metabolic lesions were found in other organs.
On August 5, 2019, radical treatment was performed on the left lobe of lung in the Shanghai Pulmonary Hospital, and postoperative pathology showed squamous cell carcinoma. Immunohistochemistry showed CK (+), VIM (+), TTF-1 (−), P40 (+), and PD-L1 (22c3) (30%). Postoperative adjuvant chemotherapy (gemcitabine + carboplatin) was administered over eight courses, and the last chemotherapy was conducted on February 10, 2020.
On February 20, 2020, the patient experienced abdominal pain. Endoscopic biopsy showed high-grade intraepithelial neoplasia of the gastric horn mucosa. Enteroscopy found protuberant lesions of rectal mucosa, and endoscopic mucosal resection (EMR) was conducted. Postoperative pathology showed rectal adenocarcinoma infiltrating half of the submucosa, with negative basal and lateral margins. All blood tumor markers were normal. Enteroscopy showed a jejunal mass (Figure 1A,1B), and biopsy pathology showed squamous cell carcinoma. PET-CT showed an irregular ring thickening of the jejunal wall, abnormal increase of glucose metabolism, and enlargement of the lymph nodes adjacent to mesenteric space (Figure 1C,1D).
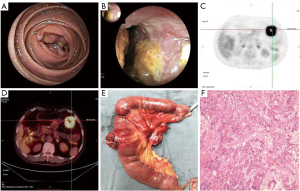
On March 10, 2020, endoscopic mucosal dissection (ESD) + laparoscopic jejunal tumor resection were performed simultaneously. Postoperative pathology of the gastric mucosal lesions showed moderately differentiated adenocarcinoma in the muscularis mucosa, negative cutting edge, and negative vessels. Postoperative pathology of the jejunal tumors showed poorly differentiated squamous cell carcinoma of the jejunum, infiltrating into the serous layer, cancer invasion of vessels and nerves, one cancer nodule in the mesentery, and no lymph node metastasis. Immunohistochemistry showed that P40 (+), CK5/6 (+), CK7 (+), CK20 (−), TTF-1 (−), PD-L1 (22c3) (60%), CDX2 (−), Ki-67 80% (+), Villin (−) (Figure 1E,1F). The postoperative diagnosis was as follows: (I) poorly differentiated squamous cell carcinoma of the jejunum; (II) early gastric cancer; (III) early rectal cancer; and (IV) postoperative squamous cell carcinoma of the left lung.
We detected a 642-gene panel NGS in four kinds of cancer tissues and blood leukocytes, and found the following: (I) the EGFR gene exhibited the same pathogenic mutation in both lung and small intestine tissues (c.2155G>Tp.G719C and c.2303G>Tp.S768I), and the mutation frequencies in the lung and small intestine were 27.5% and 26.4%, and 46.4% and 45%, respectively; (II) the mutation frequencies of the PPM1D gene (c.1787A>G:p.H596R) in the lung and small intestine were 64.6% and 78.2%, respectively; (III) FGFR4 mutation (c.1162G>A:p.G388R) was found in the blood and four tissues (jejunum, lung, stomach, rectum); and (IV) the mutation spectrum of gastric and rectal tissues was almost different from that of squamous cell carcinoma (Figure 2).
The lung and small intestine had identical EGFR gene mutation sites. The patient was diagnosed with metastatic jejunal squamous cell carcinoma and was given 8 courses of docetaxel at 120 mg each. On August 7, 2021, chest and abdominal CT was conducted and blood tumor markers were re-examined, and no tumor recurrence was observed. The patient was still alive during telephone follow-up before submission. (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional review board of Wenzhou central Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The patient was diagnosed with jejunal squamous cell carcinoma 9 months after lung cancer surgery, but we don’t know if jejunal squamous cell carcinoma is primary or metastatic. On the one hand, primary tumor was considered because there was no intestinal metastasis on PET-CT (which is more sensitive to squamous cell carcinoma) before lung cancer surgery. On the other hand, metastatic tumor was considered because lung and jejunum are squamous cell carcinomas, the interval between onset is not long, and small intestinal metastasis of lung cancer is more common than primary small intestinal squamous cell carcinoma.
It has been reported that CK7, CK20, TTF-1, and CDX2 have high sensitivity and specificity in distinguishing primary from metastatic tumors. CK7 and TTF-1 are expressed in lung tissue, while CK20 and CDX2 are mainly expressed in intestinal tissue (5); however, the above indicators can be used to judge adenocarcinoma tissue. Only CK7 positive in the patient’s small intestinal squamous cell carcinoma could not confirm that the small intestinal squamous cell carcinoma is a metastatic tumor. Therefore, we analyzed whether the gene mutation spectrum and mutation frequency of the tumors were consistent. NGS detection showed that there were high frequency EGFR gene mutations (c.2303G>T, c.2155G>T) in the lung and small intestinal squamous cell carcinomas. These two mutations were included in the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/); considering the pathogenic mutation combined with the pathogenesis sequence, they were diagnosed as metastatic jejunal squamous cell carcinoma.
This is the first reported gene mutation profile of metastatic small intestinal cancer. A rare pathogenic double site EGFR gene mutation was observed in squamous cell carcinoma. Previous reports have indicated that compared with other rare mutations in non-small cell lung cancer (NSCLC), the frequency of these two mutations may be higher (6,7), and compared with single site mutation, double site mutation has shorter a progression free survival (PFS) and a lower object response rate (ORR) (8). EGFR mutation are present in 4.5% of lung squamous cell carcinomas, and treatment with tyrosine kinase inhibitor (TKI) provide a survival benefit, but this benefit may still be lower than that of lung adenocarcinomas with EGFR mutations (8). We also found a high frequency mutation of PPM1D (c.1787A>G) in lung and small intestinal squamous cell carcinoma tissues for the first time, which were included in the exome aggregation consortium (EXAC) (http://exac.broadinstitute.org) and the Chinese 1,000 genome databases (http://www.internationalgenome.org/data). The minor allele frequency (MAF) values were g =0.000059/7 and g =0.0002/1, respectively. PPM1D is a negative regulatory gene of deoxyribonucleic acid (DNA) damage repair and a negative regulatory of the tumor suppressor gene, p53(9). It is involved in the occurrence and progression of numerous tumors, including pancreatic, colorectal, NSCLCs(10), etc. It is worth noting that PPM1D may be a potential therapeutic and prognostic target for multiple tumors. A study has shown that PD-L1 is highly expressed in the gastrointestinal metastasis of lung cancer (11). In our case, PD-L1 expression in lung cancer and small intestinal squamous cell cancer tissues was 30% and 60%, respectively.
Considering that the patient’s father had a history of colon cancer, and the patient had both gastric and rectal adenocarcinoma, we suspected whether there was a pathogenic germline gene mutation. We found FGFR4 (c.1162G>A:p.G388R) in patients’ blood leukocytes and four tumor tissue samples, which was included in the ClinVar database as variant of undetermined significance (VUS), MAF =0.3 in the 1,000 human genome database, and was common to nearly half of cancer patients. Ulaganathan et al. reported that in a mouse model of breast cancer and lung cancer carrying the mutation, this mutation changed the transmembrane structure of FGFR, exposed the signal transducer and activator of transcription 3 (STAT3) binding site in the membrane, and made STAT3 protein aggregate and phosphorylate, thereby activating the STAT3 signaling pathway and accelerating tumor progression, resulting in a worse prognosis (12).
Since the patient’s history and immunohistochemistry results could not determine the origin of the rare small intestinal squamous cell carcinoma, we examined the lung and small intestinal squamous cell carcinoma tissues by NGS, and confirmed that the small intestinal squamous cell carcinoma is a metastatic malignant tumor. We revealed the gene mutation spectrum of metastatic small intestinal squamous cell carcinoma for the first time, and identified a rare double site mutation of the EGFR gene, which has considerable diagnostic and treatment value. We also discovered, for the first time, a high frequency mutation of PPM1D gene (c.1787A>G) in lung and small intestine squamous cell carcinoma tissues, which may be involved in tumor occurrence and progression. In addition, we found that the patient carried a potentially pathogenic germline mutation in the FGFR4 gene, which may increase the patient’s risk of various cancers.
This is the first paper to analyze the gene profile of metastatic small intestinal squamous cell carcinoma. Genetic testing helped us identify the source of metastases, helped us find two rare mutations in the squamous cell carcinoma EGFR gene, and helped us find that FGFR4 (c.1162G>A:p.G388R) mutation may play an important role in tumor development. The limitations with our study including the nature of work, only one case report, and the significance of PPM1D gene and FGFR4 gene mutations is uncertain. So the significance of these mutations need to be explored in the future, and the patient need follow-up to see if other malignancies recur.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-481/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-481/coif). YW is from Zhejiang Shengting Medical Laboratory Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional review board of Wenzhou central Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu Y, Feit N, Huang Y, et al. Gastrointestinal metastasis of primary lung cancer: An analysis of 366 cases. Oncol Lett 2018;15:9766-76. [Crossref] [PubMed]
- Jevremovic V. Is gastrointestinal metastasis of primary lung malignancy as rare as reported in the literature? A comparison between clinical cases and post-mortem studies. Oncol Hematol Rev 2016;12:51. [Crossref]
- Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer 2006;42:3157-60. [Crossref] [PubMed]
- Suzuki T, Noda M, Yamamura A, et al. Persistent fecal occult blood due to the small intestinal metastasis of pleomorphic lung carcinoma. J Surg Case Rep 2022;2022:rjac043.
- Wang FD, Wang ZW, Xue HD, et al. Primary Squamous Cell Carcinoma of the Small Intestine: Pathogenesis and Clinical Features. Chin Med J (Engl) 2016;129:2131-3. [Crossref] [PubMed]
- Leventakos K, Kipp BR, Rumilla KM, et al. S768I Mutation in EGFR in Patients with Lung Cancer. J Thorac Oncol 2016;11:1798-801. [Crossref] [PubMed]
- Krawczyk P, Reszka K, Ramlau R, et al. Prevalence of rare EGFR gene mutations in nonsmall-cell lung cancer: a multicenter study on 3856 Polish Caucasian patients. Ann Oncol 2016;27:358-9. [Crossref] [PubMed]
- Barnet MB, O'Toole S, Horvath LG, et al. EGFR-Co-Mutated Advanced NSCLC and Response to EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2017;12:585-90. [Crossref] [PubMed]
- Zhu YH, Zhang CW, Lu L, et al. Wip1 regulates the generation of new neural cells in the adult olfactory bulb through p53-dependent cell cycle control. Stem Cells 2009;27:1433-42. [Crossref] [PubMed]
- Yang H, Gao XY, Li P, et al. PPM1D overexpression predicts poor prognosis in non-small cell lung cancer. Tumour Biol 2015;36:2179-84. [Crossref] [PubMed]
- Ishikawa E, Nakaguro M, Nakamura M, et al. Gastrointestinal tract metastasis of lung cancer: The PD-L1 expression and correlated clinicopathological variables. Pathol Int 2021;71:33-41. [Crossref] [PubMed]
- Ulaganathan VK, Sperl B, Rapp UR, et al. Germline variant FGFR4 p.G388R exposes a membrane-proximal STAT3 binding site. Nature 2015;528:570-4. [Crossref] [PubMed]
(English Language Editor: A. Kassem)




