
A systematic review and meta-analysis on the efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of primary liver cancer
Introduction
Primary liver cancer, referred to as liver cancer, is an epithelial malignant tumor originating from the liver (1). Primary liver cancer is the fifth most common cancer worldwide, accounting for 5.6% of all cancers (2-4). With the continuous development of medicine, there are various treatment methods for primary liver cancer, including liver resection, liver transplantation, thermal ablation, transcatheter arterial chemoembolization (TACE), radiotherapy, and systemic therapy (5-7). TACE is suitable for patients with advanced liver cancer, massive liver cancer, multi-nodular liver cancer, liver cancer recurrence after liver cancer surgery, and liver cancer rupture and hemorrhage who are unresectable and have no serious impairment of liver and kidney function. It can also be used to control local pain, bleeding, and embolism venous fistula, among other conditions (8,9).
Radiofrequency ablation (RFA) is the ablation technique that has been used for the longest time in the treatment of solid tumors (10). The principle is to puncture the radiofrequency electrode into the tumor tissue, and the ions in the tumor tissue rub and collide with each other to produce thermobiological effects under the action of a high-frequency alternating current of 375–500 kHz (11-13). When the tissue is heated to above 60 ℃, it can cause coagulation necrosis of cells.
TACE can effectively reduce tumor blood supply and reduce heat loss during RFA to enhance the therapeutic effect of RFA. At the same time, TACE can assess tumor ablation and enhance the therapeutic effect of sub-focal lesions. In recent years, RFA combined with TACE has been widely used in the treatment of primary liver cancer. Ren et al. [2019] (14) showed that RFA combined with TACE had better efficacy than RFA alone in the treatment of primary liver cancer. However, Jiang et al. [2021] (15) concluded that the combination regimen did not significantly improve patient survival compared with RFA treatment alone. Therefore, the effectiveness of combination therapy remains controversial.
In order to determine whether RFA combined with TACE has good efficacy and safety for primary liver cancer treatment, existing relevant clinical trials were innovatively and systematically screened and evaluated. It was expected that quantitative and comprehensive conclusions can be drawn, which have guiding significance for clinical application and the selection of treatment methods. We present the following article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-816/rc).
Methods
Article retrieval
The required articles were searched and retrieved from PubMed, Embase, MEDLINE, Science Direct, The Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Science and Technology Journal Database, and China Biomedical Literature Database (CBM). Relevant randomized controlled trials (RCTs) of TACE combined with RFA published from the establishment of the database to September 20, 2021 were searched. Professional journals were manually searched to avoid omissions. If the relevant data in the included articles could not be obtained from the text, the corresponding author was contacted. For the search strategy, English search keywords included transhepatic arterial chemoembolization, TACE, radiofrequency ablation, RFA, primary liver cancer, and liver tumor. Chinese search keywords included transcatheter arterial chemoembolization, TACE, radiofrequency ablation, RFA, primary liver cancer, and liver tumor. In the retrieval process, multiple retrievals were carried out in the form of free combination of keywords to obtain references that could be included, and they were tracked down using search engines.
Inclusion and exclusion criteria of articles
In order to obtain high-quality research results, PICOS principle is used to help complete the research design. P (research object): the corresponding research object should be determined according to the research objective. I/C (intervention/control measures): define the intervention measures and control measures. O (research results): the main endpoint indicators with core significance for clinical effectiveness evaluation. S (research design): determine the included research type according to the analysis goal.
The inclusion criteria were defined as follows: the research type was RCTs of TACE combined with RFA; the subjects were patients with primary liver cancer diagnosed by pathology or imaging examinations (over 18 years old); the patients in the experimental group were treated with TACE combined with RFA, and the patients in the control group were treated with RFA alone; outcome indicators included 1-, 3-, and 5-year overall survival rates, 1- and 3-year tumor-free survival rates, and the complication rates of patients with primary liver cancer.
The exclusion criteria were defined as follows: articles which were reviews, animal experiments, letters, comments, conference abstracts; patients with metastatic liver cancer; combined with other treatment methods such as immunotherapy and radiotherapy; studies with incomplete original data such as repeated publications and survival.
Outcome indicators
The outcome indicators were the 1-, 3-, and 5-year overall survival rates, 1- and 3-year tumor recurrence-free survival rates, and complication rates for primary liver cancer.
Data extraction
Two professionals used a unified Microsoft Excel (Microsoft, the United States) spreadsheet to independently screen the articles and extract data strictly according to the inclusion and exclusion criteria, and cross-checked the final results. If there was any disagreement, it could be resolved through discussion. The abstracts of the searched articles were first read, and if all the information could not be extracted, the full text was read for screening. The extracted data included: (I) basic information of the included studies, such as title, first author, publication time, country, publication journal, literature source; (II) basic characteristics of the research subjects, such as gender ratio, age, sample size of the experimental group and the control group; (III) outcome indicators, including the 1-, 3-, and 5-year overall survival rates, 1- and 3-year tumor recurrence-free survival rates, and complication rates of primary liver cancer.
Assessment of bias risk
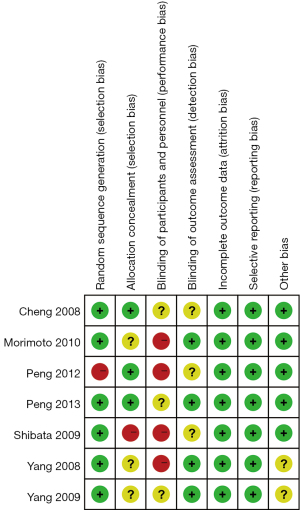
The articles included in this meta-analysis were repeatedly independently evaluated and cross-checked by 2 professionals in strict accordance with the 5 evaluation criteria of standard RCTs of the Cochrane RoB 2.0. If there was any disagreement, it could be resolved through discussion. The evaluation criteria included: (I) the random sequence generation method; (II) the concealment of the allocation scheme; (III) whether the researcher was blinded; (IV) whether there was dropout or loss to follow-up, and whether the outcome data was complete; (V) whether the number of patients in each group and their age were comparable, whether there was a selection bias, and whether there was a chance effect and its magnitude. Each entry was assessed using “low risk”, “high risk”, and “unclear risk”. The research evidence was graded A, B, and C according to the quality from strong to weak.
Statistical methods
The bias risk of the included articles was assessed using the risk of bias assessment chart of RevMan 5.3 software (Cochrane, the United States). Enumeration data were expressed as odds ratio (OR) and 95% confidence interval (CI). Heterogeneity among the articles was assessed using the chi-square test (Chi2) and the I2 test. If I2<50% and P>0.1, the degree of heterogeneity was considered to be low, and the fixed effect model (FEM) was used for combined analysis. If I2>50% and P<0.1, the degree of heterogeneity was considered to be high, and the random effects model (REM) was used for combined analysis. An inverted funnel plot was used to test for publication bias. The difference was considered to be statistically significant at P<0.05.
Sensitivity analysis
Sensitivity analysis of efficacy indicators was performed by changing the effect model (REM/FEM) to assess the reliability of the conclusions obtained.
Results
Retrieval results and basic information of the included articles
A total of 217 articles were obtained by searching databases, 25 articles were repeatedly published, 48 articles were unqualified, and 23 articles were excluded for other reasons, so the remaining 121 articles were selected. By reading the abstracts and titles, 36 articles were deleted and 85 articles were left, then 62 research reports and reviews were excluded, leaving 23 articles. After the full texts were read, 7 non-RCTs were excluded, 6 articles were excluded as the survival rate data of the studies could not be further extracted, 3 articles did not study primary liver cancer, and 7 articles were finally included in the meta-analysis. Figure 1 is a flow chart of the article retrieval process.
The quality evaluation results showed that the evaluation grade of 2 articles was A, and the evaluation grades of 5 articles were B. All 7 articles met the inclusion criteria, and there were 754 cases of liver cancer. In the 7 articles, the sample size ranged from 36 to 196 cases. All 7 articles described the 1-, 3-, and 5-year overall survival rates for primary liver cancer, 5 articles described the 1- and 3-year tumor recurrence-free survival rates, and 5 articles described the complication rates. Table 1 lists the basic characteristics of the included articles.
Table 1
| First author | Year of publication | Number of patients | Intervention | Average size of tumor (cm) | Child-Pugh grade A/B/C | Outcome indicators | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | ||||||
| Cheng (16) | 2008 | 96 | 100 | TACE + RFA | RFA | 4.96±1.34 | 4.98±1.35 | 55/41/0 | 60/40/0 | 1-, 3-, and 5-year overall survival and complication rates for primary liver cancer | |||
| Morimoto (17) | 2010 | 19 | 18 | TACE + RFA | RFA | 3.6±0.7 | 3.7±0.6 | 18/1/0 | 16/2/0 | 1-, 3-, and 5-year overall survival, 1- and 3-year tumor recurrence-free survival, and complication rates for primary liver cancer | |||
| Peng (18) | 2012 | 69 | 70 | TACE + RFA | RFA | ≤ 5 | 90/4/0 | 90/5/0 | 1-, 3-, and 5-year overall survival, 1- and 3-year tumor recurrence-free survival, and complication rates for primary liver cancer | ||||
| Peng (19) | 2013 | 94 | 95 | TACE + RFA | RFA | 3.47±1.44 | 3.39±1.35 | 60/9/0 | 59/11/0 | 1-, 3-, and 5-year overall survival, 1- and 3-year tumor recurrence-free survival, and complication rates for primary liver cancer | |||
| Shibata (20) | 2009 | 46 | 43 | TACE + RFA | RFA | 1.7±0.6 | 1.6±0.5 | 32/14/0 | 33/10/0 | 1-, 3-, and 5-year overall survival, 1- and 3-year tumor recurrence-free survival, and complication rates for primary liver cancer | |||
| Yang (21) | 2008 | 24 | 12 | TACE + RFA | RFA | 6.6±0.6 | 5.2±0.4 | ND | ND | 1-year overall survival for primary liver cancer | |||
| Yang (22) | 2009 | 31 | 37 | TACE + RFA | RFA | 1.7–7.3 | 2–6.4 | 20/10/1 | 23/13/1 | 1-, 3-, and 5-year overall survival rates and 1-year tumor recurrence-free survival rates for primary liver cancer | |||
TACE, transarterial chemoembolization; RFA, radiofrequency ablation; ND, not described.
Risk of bias assessment results of the included articles
Figure 2 and Figure 3 are the reference risk bias assessment graph and summary graph drawn by RevMan 5.3 software. Of the 7 RCTs in this meta-analysis, 6 described the generation of random sequences in detail, 3 RCTs described allocation concealment in detail, and patient blinding was not described due to different quality methods. Four articles used operator blinding, and all 7 had complete outcome measures. Except for patient blinding, all other risks of bias were low.
Meta-analysis results of 1-, 3-, and 5-year overall survival in primary liver cancer
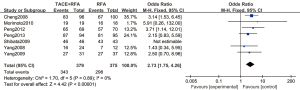
A total of 7 articles (16-22) analyzed the 1-year overall survival rate of primary liver cancer. There were 379 cases in the experimental group and 375 cases in the control group. Figure 4 is a forest plot of 1-year overall survival for primary liver cancer, analyzed using the FEM. The 1-year overall survival rates of the 7 articles showed Chi2 =1.70, degree of freedom (df) =5, I2=0%, and P=0.89, so there was no heterogeneity. The 1-year overall survival rate of the RFA combined with TACE group was significantly better than that of the RFA group, and the difference was statistically significant (OR =2.73, 95% CI: 1.75–4.26, Z=4.42, P<0.00001).
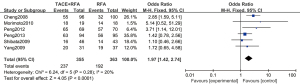
A total of 6 articles (16-20,22) analyzed the 3-year overall survival rate of patients with primary liver cancer. There were 355 cases in the experimental group and 363 cases in the control group. Figure 5 is a forest plot of 3-year overall survival for primary liver cancer. The heterogeneity test of the 3-year overall survival rates of 6 articles using the FEM showed Chi2 =6.24, df =5, I2=20%, and P=0.28, so there was no heterogeneity in each study group. The 3-year overall survival rate of the RFA combined with TACE group was significantly better than that of the RFA group, and the difference was statistically significant (OR =1.97, 95% CI: 1.42–2.74, Z=4.05, P<0.0001).
A total of 4 articles analyzed the 5-year overall survival rate of patients with primary liver cancer. There were 290 cases in the experimental group and 302 cases in the control group. Figure 6 is a forest plot of 5-year overall survival for patients with primary liver cancer, which was analyzed using the FEM. The heterogeneity analysis on the 5-year overall survival rates of 4 articles showed Chi2 =6.43, df =3, I2=53%, and P=0.09, and there was no heterogeneity in each study group. The 5-year overall survival rate of the RFA combined with TACE group was significantly better than that of the RFA group, and the difference was statistically significant (OR =2.35, 95% CI: 1.28–4.32, Z=2.74, P=0.006).
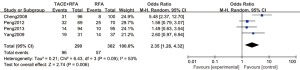
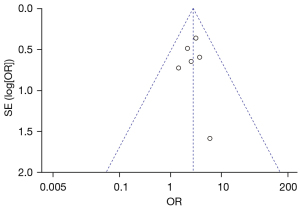
Figure 7 is a funnel plot of the 1-year overall survival rate of primary liver cancer. The circles representing the included articles were concentrated near the midline and were basically symmetrical. It was inferred that there was no publication bias in the results of this meta-analysis.
Meta-analysis of the 1- and 3-year tumor recurrence-free survival rates
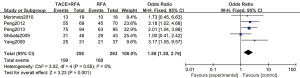
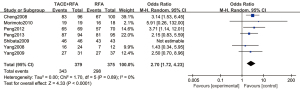
A total of 5 articles analyzed the 1-year tumor recurrence-free survival rate of patients with primary liver cancer. There were 259 cases in the experimental group and 263 cases in the control group. Figure 8 is a forest plot of the 1-year tumor recurrence-free survival rate of patients with primary liver cancer, which was analyzed using the FEM. The 1-year tumor recurrence-free survival rates of 5 articles of primary liver cancer were tested for heterogeneity, and the results revealed Chi2 =3.02, df =4, I2=0%, and P=0.55, indicating that there was no heterogeneity in each study group. The 1-year tumor recurrence-free survival rate in the RFA combined with TACE group was significantly better than that in the RFA group, and the difference was statistically significant (OR =1.88, 95% CI: 1.28–2.76, Z=3.23, P=0.001).
A total of 3 articles analyzed the 3-year tumor recurrence-free survival rate of patients with primary liver cancer. There were 209 cases in the experimental group and 208 cases in the control group. Figure 9 is a forest plot of the 3-year tumor recurrence-free survival rate for patients with primary liver cancer, which was analyzed using the FEM. The 3-year tumor recurrence-free survival rates of 3 articles were tested for heterogeneity, and the results showed Chi2 =0.24, df =2, I2=0%, and P=0.89, indicating that there was no heterogeneity in each study group. The 3-year tumor recurrence-free survival rate in the RFA combined with TACE group was significantly better than that in the RFA group, and the difference was statistically significant (OR =2.11, 95% CI: 1.37–3.24, Z=3.38, P=0.0007).
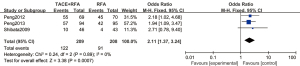
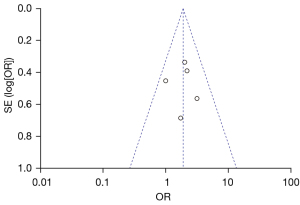
Figure 10 is a funnel plot of the 1-year tumor recurrence-free survival rate of patients with primary liver cancer. The circles representing the included articles were concentrated near the midline, which was basically symmetrical. It could be inferred that there was no publication bias in the results of this meta-analysis.
Meta-analysis of complication rates
A total of 5 articles analyzed the survival rate of patients with complications of primary liver cancer. There were 324 cases in the experimental group and 326 cases in the control group. Figure 11 is a forest plot of the complication rate of patients using the FEM. The main complications included ascites, pleural effusion, gastrointestinal bleeding, liver abscess, cholecystitis, spontaneous bacterial peritonitis, tumor cell seeding, skin burns, biliary stricture, and small bowel obstruction. The heterogeneity test was performed, and the results showed that Chi2 =4.01, df =4, I2=0%, P=0.40, indicating that there was no heterogeneity in each study group. There was no significant difference in the complication rate of patients with primary liver cancer between the RFA combined with TACE group and the RFA group (OR =0.79, 95% CI: 0.45–1.39, Z=0.81, P=0.42).
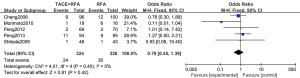
Figure 12 is a funnel plot of the complication rate of patients with primary liver cancer. The circles representing the included articles were concentrated near the midline and were basically symmetrical. It could be inferred that there was no publication bias in the results of this meta-analysis.
Sensitivity analysis
Sensitivity analysis was performed after excluding each study one by one. Figure 13 is a forest plot of the 1-year overall survival rate of patients with primary liver cancer, which was analyzed using the REM. The results showed that OR =2.70, 95% CI: 1.72–4.23, Z=4.33, and P<0.0001. The pooled effect value was still statistically significant, and the forest plot results did not change direction, indicating that the pooled effect was valid and credible.
Discussion
Primary liver cancer is a common malignant tumor with insidious onset and poor curative effect. Surgical resection is always the most ideal treatment for liver cancer (23). However, most patients are in the middle and late stages when they are diagnosed, and they are prone to liver cirrhosis, which leads to poor liver function. The tumor is close to large blood vessels, which makes surgical resection more difficult. In addition, some patients are older and have a poor general condition, so surgical treatment cannot be performed. Therefore, non-surgical treatment is suitable for most patients to improve the efficacy. With the extension of modern physics technology to the medical field, and the wide application of modern imaging technology, microelectronics, computer information processing, and other technologies in the medical field, RFA of liver cancer occupies an important place in the overall treatment mode of liver cancer, and its increasingly important role has opened up a wider opportunity for the treatment of liver cancer (24).
Scholars such as Rossi et al. [1990] (25) have conducted systematic research on RFA and significantly improved the efficacy of RFA. Under the guidance of computed tomography (CT) or ultrasound, the ablation needle is inserted into the tumor through percutaneous puncture, and the high-frequency current causes the tissue ions in the living body to vibrate in the direction of the current change, so that the tissue ions affected by the current around the electrodes rub against each other to generate heat. This results in local tissue protein denaturation, cell membrane disintegration, coagulation necrosis, and even charring, and finally complete tumor treatment. Some scholars believe that the clinical efficacy of TACE combined with RFA in the treatment of primary liver cancer is significantly superior to that of RFA alone. Kirikoshi et al. [2009] (26) observed 268 patients with primary liver cancer, and found that TACE combined with RFA treatment improved the survival rate of patients compared with TACE alone, with a statistically significant difference. However, it has also been reported that there is no significant difference in the clinical efficacy of TACE combined with RFA and RFA alone in the treatment of primary liver cancer (27-30).
This meta-analysis collected a number of high-quality RCTs to analyze and compare the clinical efficacy of TACE combined with RFA and RFA alone in the treatment of primary liver cancer, hoping to provide a reference for the selection of clinical interventional therapy for liver cancer. Seven RCTs with a total of 754 patients were included in this meta-analysis. The results of this meta-analysis showed that RFA combined with TACE could improve the 1-, 3-, and 5-year overall survival rates and 1- and 3-year tumor recurrence-free survival rates of patients with primary liver cancer, which was significantly better than that of RFA alone (P<0.05). Such results are consistent with the findings of Chen et al. [2016] (31). In addition to this, there was no significant difference in the incidence of major complications between the two groups, indicating a favorable safety profile for both combination therapy and RFA alone.
Through sensitivity analysis, it was confirmed that the conclusions obtained by applying different analysis models in this meta-analysis were consistent, and the results had good stability. The distribution of included studies in the funnel plot was symmetrical and there was no publication bias. This meta-analysis was limited by the number and level of existing clinical trials. The included articles were all RCTs, and the sample size of each study was generally small. The cases were all hospital controls, and selection bias could not be completely ruled out. In addition, due to the limitations of the objective conditions, the search scope may not cover all relevant literature, which reduced the strength of this study to a certain extent, and high-quality, large-sample RCTs are needed to confirm in the future.
Conclusions
Relevant articles on TACE combined with RFA in the treatment of primary liver cancer were screened and included in this meta-analysis, aiming to compare the efficacy and safety of combined therapy and RFA alone in the treatment of primary liver cancer. Meta-analysis results confirmed that TACE combined with RFA was safe and effective in the treatment of primary liver cancer, and can improve the overall survival and recurrence-free survival of patients with primary liver cancer. However, due to the small sample size, the test performance may be reduced. Therefore, it is necessary to further expand the sample size in terms of research design in the future, strictly abide by the standards of randomized controlled experiments, enhance the consistency between studies, and improve the quality of research, so as to obtain rigorous experimental conclusions. In conclusion, this meta-analysis showed guiding value for the treatment of primary liver cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-816/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-816/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rivera K, Jeyarajah DR, Washington K. Hepatectomy, RFA, and Other Liver Directed Therapies for Treatment of Breast Cancer Liver Metastasis: A Systematic Review. Front Oncol 2021;11:643383. [Crossref] [PubMed]
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9. [Crossref] [PubMed]
- Ji Q, Fu Y, Zhu X, et al. Effect of RFA and TACE combined with postoperative cytokine-induced killer cell immunotherapy in primary hepatocellular carcinoma. J BUON 2021;26:235-42. [PubMed]
- Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON 2019;24:163-70. [PubMed]
- Kim AR, Park E, Kwon SY, et al. Efficacy and Safety of Combined Radiofrequency Ablation with Transarterial Chemoembolization in Patients with Barcelona Clinic Liver Cancer Stage A Hepatocellular Carcinoma Ineligible for Curative Treatment. Korean J Gastroenterol 2019;73:167-76. [Crossref] [PubMed]
- Guo W, He X, Li Z, et al. Combination of Transarterial Chemoembolization (TACE) and Radiofrequency Ablation (RFA) vs. Surgical Resection (SR) on Survival Outcome of Early Hepatocellular Carcinoma: A Meta-Analysis. Hepatogastroenterology 2015;62:710-4. [PubMed]
- Yi PS, Huang M, Zhang M, et al. Comparison of Transarterial Chemoembolization Combined with Radiofrequency Ablation Therapy versus Surgical Resection for Early Hepatocellular Carcinoma. Am Surg 2018;84:282-8. [Crossref] [PubMed]
- Muhammad A, Dhamija M, Vidyarthi G, et al. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol 2013;5:364-71. [Crossref] [PubMed]
- Hirooka M, Hiraoka A, Ochi H, et al. Transcatheter arterial chemoembolization With or Without Radiofrequency Ablation: Outcomes in Patients With Barcelona Clinic Liver Cancer Stage B Hepatocellular Carcinoma. AJR Am J Roentgenol 2018;210:891-8. [Crossref] [PubMed]
- Lu B, Zhu L, Wang X, et al. Effects of radiofrequency ablation combined with transarterial chemoembolization and antiviral therapy on the prognosis and quality of life in primary chronic HBV-related liver cancer. J BUON 2019;24:1979-84. [PubMed]
- Wang YH, Liu JF, Li F, et al. Radiofrequency ablation combined with transarterial chemoembolization for unresectable primary liver cancer. Chin Med J (Engl) 2009;122:889-94. [PubMed]
- Shao Z, Liu X, Peng C, et al. Combination of transcatheter arterial chemoembolization and portal vein embolization for patients with hepatocellular carcinoma: a review. World J Surg Oncol 2021;19:293. [Crossref] [PubMed]
- Zhu K, Huang J, Lai L, et al. Medium or Large Hepatocellular Carcinoma: Sorafenib Combined with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology 2018;288:300-7. [Crossref] [PubMed]
- Ren Y, Cao Y, Ma H, et al. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer 2019;19:983. [Crossref] [PubMed]
- Jiang C, Cheng G, Liao M, et al. Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol 2021;19:81. [Crossref] [PubMed]
- Cheng BQ, Jia CQ, Liu CT, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA 2008;299:1669-77. [Crossref] [PubMed]
- Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116:5452-60. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 2012;262:689-700. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [Crossref] [PubMed]
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 2009;252:905-13. [Crossref] [PubMed]
- Yang P, Liang M, Zhang Y, et al. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Adv Ther 2008;25:787-94. [Crossref] [PubMed]
- Yang W, Chen MH, Wang MQ, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res 2009;39:231-40. [Crossref] [PubMed]
- Lan T, Chang L, Mn R, et al. Comparative Efficacy of Interventional Therapies for Early-stage Hepatocellular Carcinoma: A PRISMA-compliant Systematic Review and Network Meta-analysis. Medicine (Baltimore) 2016;95:e3185. [Crossref] [PubMed]
- Liu F, Chen M, Mei J, et al. Transarterial Chemoembolization Combined with Radiofrequency Ablation in the Treatment of Stage B1 Intermediate Hepatocellular Carcinoma. J Oncol 2019;2019:6298502. [Crossref] [PubMed]
- Rossi S, Fornari F, Pathies C, et al. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori 1990;76:54-7. [Crossref] [PubMed]
- Kirikoshi H, Saito S, Yoneda M, et al. Outcome of transarterial chemoembolization monotherapy, and in combination with percutaneous ethanol injection, or radiofrequency ablation therapy for hepatocellular carcinoma. Hepatol Res 2009;39:553-62. [Crossref] [PubMed]
- Yamagiwa K, Shiraki K, Yamakado K, et al. Survival rates according to the Cancer of the Liver Italian Program scores of 345 hepatocellular carcinoma patients after multimodality treatments during a 10-year period in a retrospective study. J Gastroenterol Hepatol 2008;23:482-90. [Crossref] [PubMed]
- Liu YM, Qin H, Wang CB, et al. Comparison of therapeutic effectiveness of combined interventional therapy for 1126 cases of primary liver cancer. World J Gastroenterol 2006;12:5060-3. [Crossref] [PubMed]
- Song MJ, Bae SH, Lee JS, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med 2016;31:242-52. [Crossref] [PubMed]
- Yuan H, Liu F, Li X, et al. Clinical efficacy of chemoembolization with simultaneous radiofrequency ablation for treatment of adrenal metastases from hepatocellular carcinoma. Cancer Imaging 2018;18:24. [Crossref] [PubMed]
- Chen QW, Ying HF, Gao S, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2016;40:309-14. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)





