
Therapeutic potential of anti-miR29a in breast cancer patients with type 2 diabetes: an in vitro and xenograft mouse-model study
Introduction
Both breast cancer and diabetes are common chronic diseases among women and are global health issues of great concern (1). Abundant epidemiological evidence shows that diabetes, especially type 2 diabetes, has been closely associated with breast cancer. The mortality rate is significantly higher among diabetic patients with breast cancer than among non-diabetic patients with breast cancer (RR =1.53; 95% CI: 1.23–1.90) (2). Previous research has shown that pre-existing diabetes is independently correlated with worse overall survival (OS) and disease-free survival (DFS) in female patients withbreast cancer (3). Type 2 diabetes can increase the synthesis of sex hormones, while high insulin levels and insulin-like growth factor (IGF) can reduce sex hormone binding-globulin (SHBG) through hepatic synthesis, activating IGF-1 and increasing androgen synthesis. Novosyadlyy et al. used MKR mice with type 2 diabetes to verify that type 2 diabetes promoted breast development and breast cancer through hyperinsulinemia (4). Breast cancer with type 2 diabetes mellitus (BDM) is provided with its unique biological characteristics and clinical characteristics.
Sirtuin 1 (SIRT1) is a type of nicotinamide adenine dinucleotide (NAD+)-dependent class III histone deacetylation (HDAC) enzyme, which is involved in energy metabolism and insulin resistance. Balestrieri et al. identified a decrease of SIRT1 in patients with diabetes (5). A SIRT1 deficiency has been shown to promote or suppress tumours in breast cancer, depending on the different molecular subtypes of breast cancer (6). Therefore, SIRT1 may be a critical determinant in the development and metastasis of breast cancer in patients with diabetes.
Several studies have proven that miRNA dysregulation is a causal factor in a variety of cancers, which play a tumour suppressors (antimiRs) or oncogenes (oncomiRs) (7,8). The antimiRs targeted miRNA mimics and molecules have shown promise in preclinical development (7). Some miRNA targeted therapies are currently being tested in clinical trials. The use of tumour suppressor miRNA miR-34 mimics has reached phase I clinical trials for the treatment of cancer, and using anti-miRs against miR-122 has reached phase II clinical trials for the treatment of hepatitis (8). MiR-29a, as a dominant member of the miR-29 family, is upregulated in the serum of type 2 diabetic patients and also in 3T3-L1 adipocytes cultured with high levels of glucose and insulin(9). MiR-29a has been shown to regulate the epithelial-mesenchymal transition (EMT) and metastasis of breast cancer cells (10). Our previous study found that breast cancer cells can undergo accelerated proliferation and metastasis due to the adjusted miR-29a expression involved in the insulin signalling pathway (11). Furthermore, recent studies have revealed that miR-29a targets the 3’untranslated region (3’UTR) of SIRT1 mRNA (12,13). Therefore, we hypothesised that anti-miR29a could be a potential target for the treatment of patients with BDM, which may be related to the activation of the SIRT1 signalling pathway. The present study investigated the contribution of anti-miR29a to MDA-MB-231 cell proliferation, migration, and invasion in BDM in vitro. It also examined the effect of anti-miR29a on tumour growth and lung metastasis in a T2DM xenograft mouse model. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-824/rc).
Methods
Clinical tissue samples
A total of 37 patients with BDM from the Third Hospital of Nanchang City (Nanchang, China) were enrolled in the study. The control group included 50 age-matched patients with breast cancer but without type 2 diabetes mellitus (NBDM) from the same period, based on an oncologist’s confirmation and a negative familial history of diabetes. All patients with primary breast cancer enrolled in this study underwent surgical resection from December 2012 to October 2016. Fresh tumour-tissue specimens were collected and immediately frozen in liquid nitrogen, then stored at −80 ℃ until needed. All the patients were female and received a postoperative histological diagnosis of invasive breast cancer. After surgery, all enrolled patients were followed up every 3 months, and their survival time was recorded. The cut-off for the follow-up period was December 31, 2019.
We recorded and compared baseline tumour characteristics for the BDM and NBDM groups (Table S1). This study was conducted in accordance with the Helsinki Declaration (as revised in 2013) and was approved by the Ethics Review Committee of the Third Hospital of Nanchang. Written informed consent was obtained from all breast cancer patients in our study.
Cell culture
MDA-MB-231 breast cancer-derived cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco BRL, Grand Island, NY, USA) containing normal glucose (5.6 mM/L), supplemented with 10% foetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, USA) at 37 ℃ in a humidified incubator with 5% CO2-95% air.
Plasmids, lentivirus production, and transduction
We purchased miRZip-29a, an shRNA-miR-29a structure cloned in pGreenPur™ lentivector, from System Biosciences (Cat No. MZIP29a-PA-1; Palo Alto, CA, USA). Lentivirus used to stably overexpress miR-29a in breast cancer cells was purchased from the Genechem Company (Shanghai, China), and the Scr control plasmid was a generous gift from Professor FC Zhang from the Ruijin Hospital of Shanghai Jiaotong University.
Either miRZip-29a plasmid or Scr control plasmid was transfected into 293T cells using Lipofectin Reagent (Invitrogen) according to the manufacturer’s instructions. Pseudoviral particles were applied to MDA-MB-231 cells. After 24 hours, the virus-infected cells were subjected to drug selection (1 µg/mL puromycin) for 7 days to achieve stable cell subclones. The subclones were labelled as blank, anti-miR29a, or vector and grouped accordingly. SIRT1 plasmid (5'-CCGGATTGAAGAATGTTGG-3', 5'-ATCTGCTCCTTTGCCACTC T-3') was cloned into a pcDNA3.1 vector to increase the expression of SIRT1 in MDA-MB-231 cells (labelled as pc-SIRT1 and pcDNA3.1). After drug-selection, cells were cultured in vitro under normal conditions (5.6 mM/L glucose + 5 nM/L insulin) or BDM conditions (25 mM/L glucose + 25 nM/L insulin) for the different experiments.
TaqMan miRNA analysis
Total RNA was extracted from the tissues and cells using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions. The miRNAs were detected by real-time reverse transcriptase polymerase chain reaction (qRT-PCR). The cDNAs were produced by reverse transcription kits (Takara, Dalian, China) and miRNA-specific bulge-loop™ miRNA RT primers (Ribobio Co, Guangzhou, China) (Table S2). We performed qRT-PCR by a StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) and an SYBR Green I Real-Time PCR kit (TaKara, Dalian, China). We used the comparative delta CT (2−∆∆Ct) method to estimate the relative expressions of miR-29a. U6 small nuclear 2 (RNU6) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as endogenous controls in this study.
Western blot
Proteins collected from the relevant cells were separated using electrophoresis on a polyacrylamide gel and transferred to a PVDF membrane. Then, 5% non-fat dry milk was used to seal the membrane in TRIS-buffered saline with Tween-20 (TBST) for 1 hour. The primary antibodies directed against SIRT1 (DF6033, Affinity Biosciences, USA) were applied to the cell membrane at 4 ℃ overnight and then washed off with TBST. After that, the membrane was incubated with a secondary antibody diluted 1:10,000. A Clarity Western enhanced chemiluminescence ECL substrate (Bio-Rad, Hercules, CA, USA) was used for protein detection. The relevant data were analysed using GraphPad Prism 5 software (GraphPad software, San Diego, CA, USA). The Western blots were repeated at least three times.
Immunohistochemistry (IHC)
For IHC analysis, paraffin sections were treated with a primary antibody against SIRT1 (1:50 dilution, DF6033, Affinity Biosciences, USA) at 4 ℃ overnight. After incubating with a secondary antibody and 3,3'-Diaminobenzidine (DAB) substrate for visualisation, the sections were subjected to fluorescence microscopy (CKX53, Olympus) for imaging.
Luciferase reporter assay
The wild-type 3'UTR of SIRT1 mRNA and the mutant type 3'UTR of SIRT1 mRNA (UGGUGCU to ACCACGA) were amplified and inserted into pmiRGLO vectors (Promega Corporation, Madison, WI, USA). The HEK-293T cells were co-transfected with the reporter plasmids and miR-29a mimics or miR-NC using Lipofectamine 3000 (Invitrogen). After transfection and incubation for 48 hours, the cells were collected, and Renilla luciferase activity was detected using the Dual Luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA).
Cell counting kit-8 assays
The cells of different groups were seeded in 96-well plates and cultured for 12, 24, 48, or 72 hours. Cell proliferation was detected using a cell counting kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) as per the manufacturer’s instructions. Absorbance at 450 nm was examined using a microplate reader (Bio-Rad, Berkeley, CA, USA). Independent experiments were performed in triplicate.
Flow cytometry analysis
All the cells were cultured in DMEM with insulin (25 nmol/L) plus high glucose (25 mmol/L) for 48 hours, then harvested and washed twice with phosphate-buffered saline (PBS) and fixed overnight at −20 ℃ with 70% ethanol. After being washed again with PBS, the cells were stained with 5 µL of propidium iodide solution (10 µg/mL) and 100 µL of RNase (100 µg/mL) in PBS and incubated for 30 minutes at room temperature. The analysis was performed using FACS Calibur (Becton Dickinson, Franklin Lakes, NJ, USA) and CellQuest Pro software (Becton Dickenson).
Wound healing assay
To determine MDA-MB-231 cell migration, the different groups of those cells were seeded onto 6-well plates and cultured under BDM conditions overnight. Wounds were made by gently scraping with a disposable pipette tip, and the MDA-MB-231 cells were photographed using a phase-contrast microscope (Olympus, Japan) at 0 and 48 h. Distance migration was measured by calculating the area of the wound using ImageJ software and represented in the form of a bar graph. All experiments in this study were repeated independently three times with similar results.
Invasion assay
Cell migration assays were performed in 24-well Transwell chambers (8.0 µm; Corning Incorporated, Corning, NY, USA) to detect the migration and invasion of the transfected cells. Every 200 µL of cell suspension was pre-coated with 50 mL of 1.0 mg/mL Matrigel (Millipore, Billerica, MA, USA), which was obtained from serum-free media with a density of 2×104 cells/mL and seeded into the upper chamber of the Transwell system. A chemo-attractant (600 µL of medium containing 10% serum) was added to the lower chamber before 24 hours of incubation. The cells on the upper side of the Transwell were removed with a cotton swab and fixed with 100% methanol (30 minutes), then stained using Crystal Violet Staining Solution (Beyotime Institute of Biotechnology).
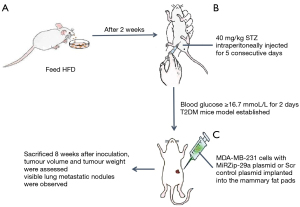
Xenograft mouse model for BDM
This animal study was approved by the Ethics Committee for animal experimental research of the Third hospital of Nanchang, and was conducted in compliance with institutional guidelines for the care and use of animals. Twenty 4-week-old female BALB/c mice were purchased from the Animal Research Center of Shanghai Jiaotong University. The mice were fed a high-fat diet (HFD) containing 60 kcal % fat ad libitum for 10 weeks and acclimated to these mice for at least 7 days before the experiment. After 2 weeks on the HFD, the mice were intraperitoneally injected for 5 consecutive days with a small dose (40 mg/kg) of streptozotocin (STZ) (Sigma-Aldrich, Beijing, China) dissolved in 100 µL of 0.05 M citrate buffer (pH 4.5). The STZ solution was prepared a few minutes before use. Three days after the administration of STZ, the mice’s random blood glucose levels were detected. The blood glucose of 12 mice was higher than 16.7 mmol/L for 2 days, indicating that the T2DM mouse model had been established successfully.
MDA-MB-231 cells with either Scr control plasmid or MiRZip-29a plasmid were washed with Dulbecco’s PBS (DPBS) and resuspended in Matrigel (10 mg/mL). The 12 mice were divided into 2 groups and received subcutaneous injections of 0.1 mL cell suspension (5×105 cells/mL) of either MDA-MB-231 cells with Scr control plasmid cells or MiRZip-29a plasmid cells in their fourth mammary fat pad. The mice were sacrificed 8 weeks after inoculation. Tumour volumes were calculated using the following formula: volume =0.5 LS2 and were assessed by measuring the longest (L) and shortest (S) diameter of the tumour under a microscope. The number of tumour nodules in the lungs was also counted after 8 weeks. Each tumor tissue was excised and embedded in paraffin for histopathological examination (Figure 1).
Statistical analyses
We used t-tests, Mann-Whitney U tests, bidirectional analysis of variance (ANOVA), and correlation analysis to compare the differences between variables in this study. Kaplan-Meier analysis was performed to compare disease-specific survival (DSS) and recurrence-free survival (RFS) between the groups. A P value <0.05 was considered statistically significant. All analyses were performed using SPSS version 18.0 software (IBM, Chicago, IL, USA). Data is shown as mean ± SD.
Results
MiR-29a is upregulated in BDM and correlated with poor prognosis
The expression of mi-29a in breast cancer cell lines and tumour tissues was measured by qRT-PCR. The expression of miR-29a was higher in BDM tissues than in NBDM tissues (Figure 2A). The expression of miR-29a in breast cancer cell lines cultured under BDM conditions was also higher than in those cultured under normal conditions (Figure 2B).
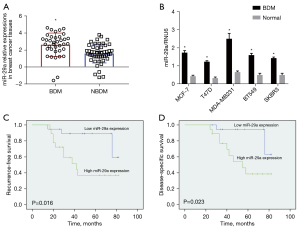
A higher expression of miR-29a was correlated with the clinicopathological characteristics of BDM. In addition, high levels of miR-29a expression were significantly correlated with the proliferative index (ki-67 ≥30%), distant metastasis, and lymph node metastasis (Table 1). Kaplan-Meier analysis suggested that patients with BDM and high miR-29a expression had significantly worse RFS and DSS than those with BDM and low miR-29aexpression (Figure 2C,2D).
Table 1
| Variable | Case, n | miR-29a-3p expression, n | P value | |
|---|---|---|---|---|
| Low (n=19) | High (n=18) | |||
| Menopausal status | ||||
| Pre-menopausal | 18 | 11 | 7 | 0.248 |
| Post menopausal | 19 | 8 | 11 | |
| Histologic type | ||||
| Special type | 6 | 4 | 2 | 0.357 |
| Non special type | 31 | 15 | 16 | |
| Axillary lymph node status | ||||
| Metastasis | 14 | 4 | 10 | 0.031 |
| No | 23 | 15 | 8 | |
| Tumor size | ||||
| ≥2 cm | 23 | 10 | 13 | 0.219 |
| <2 cm | 14 | 9 | 5 | |
| ER | ||||
| Positive | 25 | 11 | 14 | 0.197 |
| Negative | 12 | 8 | 4 | |
| PR | ||||
| Positive | 21 | 10 | 11 | 0.603 |
| Negative | 16 | 9 | 7 | |
| HER-2 | ||||
| Negative | 27 | 12 | 15 | 0.312 |
| Positive | 10 | 7 | 3 | |
| Ki67 | ||||
| ≥30% | 20 | 6 | 14 | 0.005 |
| <30% | 17 | 13 | 4 | |
| Distant metastasis | ||||
| Yes | 12 | 3 | 9 | 0.026 |
| No | 25 | 16 | 9 | |
ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
Anti-miR-29a inhibited cell proliferation in BDM
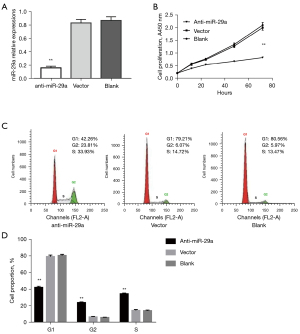
To determine the effect of miR-29a knockdown on the proliferation of breast cancer cells in BDM, we constructed an antisense miR-29a vector (“shRNA-miR-29a”, referred to as “anti-miR-29a”). We established stable MDA-MB-231 cells constitutively expressing “anti-miR-29a” by utilising this vector. Our qRT-PCR assay showed that miR-29a levels decreased significantly in the stable cells (Figure 3A). A CCK-8 assay showed the cell proliferation abilities in the anti-miR-29a group sharply decreased relative to the vector and blank control groups (Figure 3B). Flow cytometric analysis indicated that miR-29a knockdown in MDA-MB-231 cells could significantly downregulate G1 phase ratios and increase G2 phase ratios (Figure 3C,3D). These data suggested that miR-29a knockdown of breast cancer cells in BDM could inhibit cell growth and proliferation.
Anti-miR-29a reduced cells migration and invasion in BDM condition
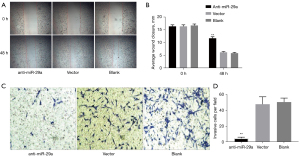
To investigate the role of miR-29a knockdown on the migration and invasive potential of MDA-MB-231 cells, we performed a wound-healing assay and a Transwell invasion assay. The wound-healing assay showed that MDA-MB-231 cells in the anti-miR-29a group displayed lower migration ability (4.67±0.33) than those in the vector control group (10.17±0.17) and blank control group (10.83±0.17) (Figure 4A,4B). The Transwell invasion assays showed that the invasion cell numbers of MDA-MB-231 cells in the anti-miR-29a group was 4.67±0.88, significantly less than in the vector control group (47.67±5.44) and the blank control group (50.33±2.91) (Figure 4C,4D). These data suggested that miR-29a knockdown of breast cancer cells in BDM reduced cell invasion and migration.
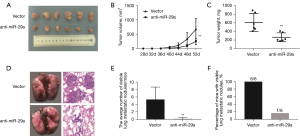
Anti-miR-29a suppresses breast cancer tumorigenesis and metastasis in T2DM mice
A diabetic mouse model was created through an HFD plus STZ. The body weight and fasting blood glucose level of all the mice in the seventh week were recorded (Table S3). Subsequently, MDA-MB-231 cells with anti-miR-29a and vector-transfected cells were implanted into the fourth mammary fat pads of these diabetic mice (n=6 in each group). These mice were followed for 8 weeks post-transplantation, and tumour volumes and weights were calculated. Interestingly, tumour growth in the anti-miR-29a group was noticeably less than in the vector control group (Figure 5A-5C). Metastatic activity was detected by examining the organs at necropsy. Histological analysis of the lungs isolated at necropsy, only one visible lung metastatic nodule was observed in the anti-miR-29a group, whereas visible lung metastatic nodules were observed in all of the vector control group mice, and 83.3% of them had multiple [5–6] metastases (Figure 5D-5F). This showed that BDM mice with miR-29a knockdown had less lung metastasis than those with the vector-transfected cells. Together, these results indicated that miR-29a knockdown suppressed MDA-MB-231 cells, tumorigenesis, and metastasis in the BDM mice.
SIRT1 is a direct target of miR-29a in BDM
Based on analysis using data from miRNA online databases, including TargetScan Human7.2 and miRBase, we found SIRT1 to be a predicted target of miR-29a (Figure 6A). To test the direct interaction between miR-29a and the 3'UTR of SIRT1, we performed a dual-luciferase reporter assay using HEK-293T cells. Not unexpectedly, a significant reduction in luciferase activity was observed in the miR-29a-overexpressing cells with wild-type reporter genes, while no difference was noted with the mutant reporter genes (Figure 6B).
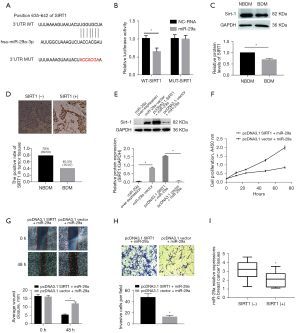
To assess the significance of SIRT1 expression in BDM, Western blot analysis was carried out on breast cancer cells, and IHC was performed on tumour tissues. Interestingly, SIRT1 protein expression in the MDA-MB-231 cells decreased by 33% after being cultured for 72 hours under BDM conditions relative to those cultured under normal conditions (Figure 6C). The IHC results showed that the positive rate of SIRT1 in BDM tissues was significantly lower than in NBDM tissues (P<0.05, Figure 6D).
To investigate the effects of miR-29a on SIRT1 expression in MDA-MB-231 cells cultured under BDM conditions, we performed the rescued experiments that miR-29a-overexpressing MDA-MB-231 cells were transfected with pcDNA3.1- SIRT1 plasmid. The results showed that protein expressions of SIRT1 were sharply downregulated in the miR-29a-overexpressing group compared with the vector control group and were remarkably restored after transfection with pcDNA3.1- SIRT1 plasmid (Figure 6E). Furthermore, the proliferation, migration, and invasion abilities of the MDA-MB-231 cells increased noticeably in the miR-29a+SIRT1 group compared with the miR-29a group (Figure 6F-6H). We also found that positive expressions of SIRT1 in BDM tissues were associated with miR-29a levels (Figure 6I). Considering these results, we demonstrated that SIRT1 might be a direct target gene of miR-29a, and that mediated SIRT1 in human BDM.
Discussion
Type 2 diabetes has been identified as an independent risk factor for women with breast cancer (14). Wei et al. found that MCF-7 cells under high glucose and high insulin conditions promoted the cells’ proliferation and invasion through activating the Ras/Raf/ERK pathway and upregulating IRS1 (15). Recent studies have revealed that patients with BDM have a greater risk of death, tend to present at later stages, and receive altered treatment regimens(16,17). In addition, compared with their non-diabetic counterparts, patients with BDM have been shown to have a 51% shorter OS time and a 28% shorter DFS (3). Therefore, it is an important clinical challenge to find a more reasonable scheme and accurate target for treating patients with BDM. High MiR-29a expression levels have been found in the serum of patients with type 2 diabetes and in 3T3-L1 adipocytes cultured with high glucose and high insulin(9). At the same time, MiR-29a is upregulated, and tristetraprolin is downregulated in patients with metastatic breast cancer, including those with invasive ductal carcinoma tissues and invasive cell lines (10,18). MiR-29a is a tumour activator and induces breast cancer cell proliferation and EMT by targeting TET1 (19). MiR-29a also promotes EMT, migration, and invasion of breast cancer cells by downregulating histone H4K20 trimethylation (20). Our previous study found that miR-29a was overexpressed in MCF-7 and T47D cells cultured in a high-insulin medium and promoted the cell growth and invasion involved in the insulin signalling pathway (11). The present study showed that miR-29a is upregulated in the tumour tissues of patients with BDM and breast cancer cell lines cultured under BDM conditions. The Kaplan-Meier analysis also confirmed that miR-29a was associated with a poor prognosis for patients with BDM. In short, our results and previous studies strongly suggest that miR-29a promotes breast cancer growth and progression under BDM conditions and could be a promising target for treating patients with BDM.
SIRT1 is often described as a “regulator of regulators” and has been reported to play an important role in glucose metabolism, DNA repair, genome maintenance, and cancer development (5,6). SIRT1 is the selective modulator in breast carcinogenesis and may act as a tumour suppressor or promoter (6). Our data indicated that SIRT1 was downregulated in BDM. According to analyses of biological information and previous research, SIRT1 is the target of miR-29a (12,13). It is upregulated in MDA-MB-231 cells and promotes tumour invasion (21). We demonstrated that miR-29a promoted cell proliferation and invasion in BDM by targeting SIRT1. This may be a novel regulatory mechanism in BDM.
As mentioned above, miR-29a plays the role of an oncogene in BDM. Therefore, miR-29a knockdown could be a promising therapeutic strategy for this subtype of breast cancer. Several studies have suggested that miR-29a knockdown promotes apoptosis of MCF-7 cells cultured in normal RPMI-1640 medium (22) or in DMEM (23), indicating that miR-29a may be a promising targeted strategy for interfering in tumour cell apoptosis. The present results showed that miR-29a knockdown inhibited breast cancer cell growth and proliferation, reducing cell invasion and migration in BDM.
Following the methods reported in previous studies (24,25), type 2 diabetes was induced in female mice before MDA-MB-231 anti-miR-29a cells or MDA-MB-231 control vector cells were injected into their mammary fat pads. As expected, the anti-miR-29a inhibited tumour growth and reduced total tumour volume and weight in these BDM xenografts. Interestingly, this treatment also reduced visible lung metastatic nodules in the BDM xenografts. Many nanoliposomes encapsulating the RNAi strategy have been used in clinical trials for several cancer types (26), showing that targeting miR-29a with anti-miRNAs as a novel therapy for the treatment of BDM is worth further exploration.
Conclusions
Our results showed that high miR-29a expression in patients with BDM was associated with a poor prognosis. MiR-29a promoted cell proliferation and invasion in BDM by targeting SIRT1. Anti-miR-29a inhibited breast cancer cell growth and proliferation and reduced cell invasion and migration in BDM. It also reduced tumour growth in the BDM xenografts. Therefore, targeting miR-29a with anti-miRNAs could be a novel therapy for the treatment of BDM and, as such, is worth further exploration.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-824/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-824/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-824/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Helsinki Declaration (as revised in 2013) and was approved by the Ethics Review Committee of the Third Hospital of Nanchang. Written informed consent was obtained from all breast cancer patients in our study. The animal study was approved by the Ethics Committee for animal experimental research of the Third hospital of Nanchang, and was conducted in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen B, Li J, Chi D, et al. Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer. Cells 2019;8:1638. [Crossref] [PubMed]
- Hashimoto Takigami N, Kuniyoshi S, Miki Y, et al. Breast Cancer, Diabetes Mellitus and Glucagon-Like Peptide-1 Receptor Toward Exploring Their Possible Associations. Breast Cancer Res Treat 2021;189:39-48. [Crossref] [PubMed]
- Wang T, Farvid MS, Kang JH, et al. Diabetes Risk Reduction Diet and Survival after Breast Cancer Diagnosis. Cancer Res 2021;81:4155-62. [Crossref] [PubMed]
- Novosyadlyy R, Lann DE, Vijayakumar A, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 2010;70:741-51. [Crossref] [PubMed]
- Balestrieri ML, Servillo L, Esposito A, et al. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia 2013;56:162-72. [Crossref] [PubMed]
- Rifaï K, Idrissou M, Penault-Llorca F, et al. Breaking down the Contradictory Roles of Histone Deacetylase SIRT1 in Human Breast Cancer. Cancers (Basel) 2018;10:409. [Crossref] [PubMed]
- Landrier JF, Derghal A, Mounien L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019;8:859. [Crossref] [PubMed]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203-22. [Crossref] [PubMed]
- He A, Zhu L, Gupta N, et al. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol 2007;21:2785-94. [Crossref] [PubMed]
- Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 2009;10:400-5. [Crossref] [PubMed]
- Li ZH, Xiong QY, Xu L, et al. miR-29a regulated ER-positive breast cancer cell growth and invasion and is involved in the insulin signaling pathway. Oncotarget 2017;8:32566-75. [Crossref] [PubMed]
- Zhang Y, Yang L, Wang S, et al. MiR-29a suppresses cell proliferation by targeting SIRT1 in hepatocellular carcinoma. Cancer Biomark 2018;22:151-9. [Crossref] [PubMed]
- Nan P, Niu Y, Wang X, et al. MiR-29a function as tumor suppressor in cervical cancer by targeting SIRT1 and predict patient prognosis. Onco Targets Ther 2019;12:6917-25. [Crossref] [PubMed]
- Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer 2012;19:793-803. [Crossref] [PubMed]
- Wei ML, Duan P, Wang ZM, et al. High glucose and high insulin conditions promote MCF-7 cell proliferation and invasion by upregulating IRS1 and activating the Ras/Raf/ERK pathway. Mol Med Rep 2017;16:6690-6. [Crossref] [PubMed]
- Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 2011;29:40-6. [Crossref] [PubMed]
- Li Z, Luo Y, Gong Y, et al. Clinical features and molecular phenotypes of breast cancer in patients with type-2 diabetes mellitus. Asian Pac J Cancer Prev 2011;12:2183-8. [PubMed]
- Al-Ahmadi W, Al-Ghamdi M, Al-Souhibani N, et al. miR-29a inhibition normalizes HuR over-expression and aberrant AU-rich mRNA stability in invasive cancer. J Pathol 2013;230:28-38. [Crossref] [PubMed]
- Pei YF, Lei Y, Liu XQ. MiR-29a promotes cell proliferation and EMT in breast cancer by targeting ten eleven translocation 1. Biochim Biophys Acta 2016;1862:2177-85. [Crossref] [PubMed]
- Wu Y, Shi W, Tang T, et al. Correction: miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via downregulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis 2019;10:856. [Crossref] [PubMed]
- Chung YR, Kim H, Park SY, et al. Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype. Hum Pathol 2015;46:1027-35. [Crossref] [PubMed]
- Khamisipour G, Mansourabadi E, Naeimi B, et al. Knockdown of microRNA-29a regulates the expression of apoptosis-related genes in MCF-7 breast carcinoma cells. Mol Clin Oncol 2018;8:362-9. [PubMed]
- Choghaei E, Khamisipour G, Falahati M, et al. Knockdown of microRNA-29a Changes the Expression of Heat Shock Proteins in Breast Carcinoma MCF-7 Cells. Oncol Res 2016;23:69-78. [Crossref] [PubMed]
- Omaña-Molina M, Sanchez-Rocha R, Hernandez-Martinez D, et al. Type 2 diabetes mellitus BALB/c mice are more susceptible to granulomatous amoebic encephalitis: Immunohistochemical study. Exp Parasitol 2017;183:150-9. [Crossref] [PubMed]
- Sanchez-Zamora Y, Terrazas LI, Vilches-Flores A, et al. Macrophage migration inhibitory factor is a therapeutic target in treatment of non-insulin-dependent diabetes mellitus. FASEB J 2010;24:2583-90. [Crossref] [PubMed]
- Tatiparti K, Sau S, Kashaw SK, et al. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials (Basel) 2017;7:77. [Crossref] [PubMed]


