
Allogeneic CAR-T bridging to allo-HSCT as a treatment strategy for refractory adult Burkitt’s lymphoma: a case report
Introduction
Burkitt’s lymphoma (BL) is a rare and highly aggressive non-Hodgkin’s lymphomas (NHL), which is considered to exist in three epidemiologically different forms, affecting different populations (1). This endemic form associated with the Epstein-Barr virus (EBV) was first described by Denis Burkitt in African children 50 years ago. Immunodeficiency-associated BL is thought to be related to HIV infection. Meanwhile, sporadic BL is relatively rare, accounting for less than 3% of all NHL, affecting children and young people in all parts of the world. The most common sites involved in sporadic BL are gastrointestinal tract, especially in ileum and cecum (1). Primary cervical BL is extremely rare and rarely reported in literature (2). At the time of diagnosis, a small number of women with extra nodal lymphoma were confined to the cervix, not involving myometrium of uterus, and there is no evidence of bone marrow invasion, which is called primary lymphomas of the cervix (LUCX) (2). Due to the rarity of the disease, comparative clinical trials are lacking and the optimal treatment strategy is not clear.
BL derived from a germinal center B cell is characterized by the activation of MYC gene of all subtypes (3). The cure rates of most BL cases has been significantly improved due to the intensive multi-drug treatment schemes. However, relapsed or refractory BL progresses rapidly, and it is associated with poor outcomes (4). Despite intensive treatment, similar to the treatment for acute lymphoblastic leukemia, few patients could survive. At present, clinical data have confirmed that CAR-T cell therapy is effective in treating B-cell malignant tumors. Our center reports that sequential infusion of CAR19/22 T cells was safe and effective for patients with recurrent or refractory B cell malignant tumors (5). In adult BL patients, further in-depth clinical study found that if the CAR-T cell therapy cannot achieve the optimal effect, it is necessary to be transferred to allogeneic hematopoietic stem cell transplantation (allo-HSCT) immediately (6).
Here, we describe a case of primary refractory BL of the uterine cervix. After receiving the CD 19/CD 22 dual-targeted CAR-T cell therapy from donors in our hospital, the patient achieved partial remission. Subsequently, she successfully received allo-HSCT treatment. Unfortunately, our patient eventually died of complications related to allo-HSCT. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-174/rc).
Case presentation
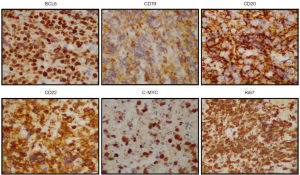
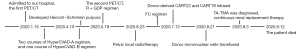
In January, 2020, a 47-year-old Chinese female patient was admitted to a local hospital for unexplained vaginal bleeding. Cervical mass was detected by ultrasonography. Cervical biopsy and immunohistochemical (IHC) analysis were performed. IHC staining of BCL6, CD20, CD19, CD22 and C-MYC revealed a positive reaction, and IHC staining of Ki67, an index related to tumour proliferation capacity, showed significant positivity (Figure 1). It was diagnosed as invasive NHL. In the middle of January, bone marrow biopsy and positron emission tomography-computed tomography (PET-CT) examination were completed in our hospital (Figure 2A), and the patient was diagnosed as primary BL of the uterine cervix at stage II with mutation of TP53 gene, MYC gene and DDX3X gene. EBV was undetectable in her peripheral blood. The patient had no history of tuberculosis, alcohol or tobacco consuming. And the patient had a normal delivery after full-term pregnancy, with no history of gynecology and obstetrics. She received three courses of chemotherapy, including two courses of HyperCVAD-A chemotherapy (including fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and one course of HyperCVAD-B chemotherapy (including methotrexate, cytarabine and methylprednisolone). After each chemotherapy cycle, the patient developed grade III agranulocytosis in the next few days. Fortunately, the number of white blood cells gradually recovered after receiving timely hematopoietic growth factor support therapy with granulocyte colony stimulating factor (G-CSF). In addition, during the last course of chemotherapy, the patient Henoch-Schönlein purpura. After symptomatic treatment, the patient’s symptoms improved and she was recuperated outside the hospital.
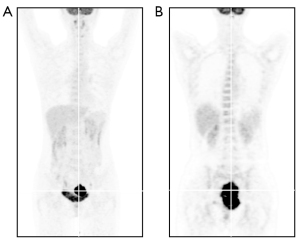
In mid-May, 2021, our patient was hospitalized again for intermittent vaginal bleeding. The result of the second PET-CT examination indicated the progress of her disease (Figure 2B). The mass of the lymph nodes on the left side of the original cervix and the pelvic wall of the original left side were larger than that before, and the metabolism of these lymph nodes was higher than before. Give GDP chemotherapy in time, including gemcitabine, cisplatin, dexamethasone, and anti-CD20 monoclonal antibody rituximab. The BCL-2 inhibitor, venetoclax, was also been given 800 mg daily to prevent the tumor from progressing. Unfortunately, there was no tendency for cervical mass to reduce, and malignant ascites appeared.
With the consent of the patient and her family, allogeneic CAR-T therapy was arranged after pelvic local radiotherapy. Our patient’s brother is the human leukocyte antigen (HLA)-matched sibling donor. In the middle of June, the patient received pelvic radiotherapy. Meanwhile, anti-CD19 CAR-T cells (CART19) and anti-CD22 CAR-T cells (CART22) were prepared with T cell derived from the donor (5,7).
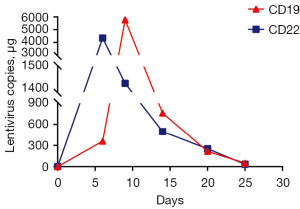
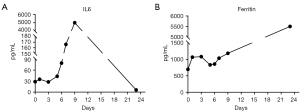
After pretreatment with FC regimen (fludarabine 30 mg/m2, day 1 to day 3; cyclophosphamide 20 mg/kg, day 1 to day 3), donor-derived CD22-targeted T cells (2×106 cells/kg) was infused on day 0 and day +1, followed by CART19 (2×106 cells/kg) on day +2 and day +3. In the peripheral blood detected by droplet digital polymerase chain reaction (ddPCR), the copies of CART22 and CART19 increased dramatically and reached the peak on the tenth day after the first infusion of donor-CAR-T cells (Figure 3). In the next few weeks, her body temperature was monitored as well as inflammatory markers such as plasma IL-6 concentration and ferritin (Figure 4A,4B). From day +5 to day +9, the patient developed fever with the highest temperature of 38.5 ℃. On the 9th day, the peak concentration of plasma IL-6 reached over 5,000 pg/mL, and on the 23rd day, the peak value of ferritin was 5,506.1 µg/L. We considered that the patient had cytokine release syndrome (CRS). The use of nonsteroidal anti-inflammatory drugs could reduce the body temperature of patient and improve the symptoms of chest tightness. As maintenance therapy, the patient has been treated with venetoclax, as a bridge to a formal identical allo-HSCT from her brother.
Before myeloablative treatment with busulfan (BU)/cyclophosphamide/antithymocyte globulin (ATG) regimen, our patient’s condition assessment obtained partial remission. She received allogeneic transplantation in August, in which G-CSF mobilized peripheral blood stem cells from her fellow donor. Infusion of donor mononuclear cells was 24.58×108 cells/kg, in which CD34 positive cells were 4×106 cells/kg. Then, our patient received graft-versus-host disease (GVHD) prevention of tacrolimus. While waiting for neutrophils and platelet to be implanted, the patient developed continuous fever. Carbapenem resistant Klebsiella pneumoniae was detected by pathogen detection, and strong anti-infection treatment was given immediately. High-throughput gene detection found that BK virus and cytomegalovirus (CMV) were present in peripheral blood samples, while EBV was always negative. Subsequently, Escherichia coli was found in peripheral blood culture. According to the test results, anti-infective drugs were adjusted in time to control the patient’s temperature.
On the 13th day after stem cell infusion, the patient developed disturbances of consciousness, accompanied by subcutaneous bleeding spots on her limbs. Routine blood examination showed thrombocytopenia, and red blood cell fragments could be seen in the peripheral blood smear. Further examination showed that the serum lactate dehydrogenase (LDH) levels increased and renal insufficiency, while Coombs’ test was negative. Based on the above results, we considered the diagnosis of transplantation related thrombotic microangiopathy (TA-TMA) in the patients as a complication after allo-HSCT treatment (8). When considering the diagnosis of TA-TMA, tacrolimus, the calcineurin inhibitor, was immediately abandoned as possible inducing factor. Continuous renal replacement therapy (CRRT) was applied as quickly as possible, while the antimicrobial therapy was continuing. Sadly, the patient’s condition was not effectively controlled, and finally she did not survive (Figure 5).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Our manuscript introduced a patient diagnosed as primary BL of the cervix, who had adverse molecular genetic changes and received multi-line chemotherapy, but failed to get remission. As a case of refractory BL, after careful consideration, the patient decided to receive donor-derived CD19/CD22 dual-targeted CAR-T therapy, and then to receive allo-HSCT. After dual-targeted CAR-T treatment, the patient got partial remission and successfully received allo-HSCT treatment. Unfortunately, while waiting for neutrophils and platelet to be implanted, our patient developed severe infections and died of complications related to HSCT.
Considering that lymphoma originated from cervical stroma, cervical biopsy and IHC analysis are needed to diagnose LUCX (9). The difficulty lies in the lack of standardization of LUCX management due to its rarity. We know that the prognosis of sporadic BL is difficult to achieve a good prognosis. Especially in patients with refractory diseases, the survival rate is obviously decreased. Some retrospective studies have shown that conventional treatments such as chemotherapy, autologous or allogeneic HSCT did not show good therapeutic effects (4). In recent years, CAR-T cell therapy has become a hot spot in the treatment of hematological diseases, and it is considered as one of the most promising approaches for chemotherapy-refractory or multiply-relapsed lymphoma (10). CD19/CD22 dual-targeted CAR-T cell therapy provides a better treatment method for reducing antigen escape relapse, and has a good antitumor effect on recurrent or refractory B- cell malignancies (5). Previous clinical study in our center has found that autologous CAR-T cell therapy may be less effective for BL than for diffuse large B-cell lymphoma, while some of these BL patients with bulky disease even failed to respond to CAR-T cells (6). Autologous T cell-derived CAR-T cell therapy is ineffective in some lymphoma patients, which is considered to be related to autologous T cell dysfunction (11). The biological characteristics of autologous T cells may be affected by many aspects, such as the various immunosuppressive mechanisms from tumor microenvironment and the negative effects of previous treatments. Meanwhile, the quality and quantity of CAR-T cells from healthy donors can be guaranteed. In view of the patient’s myelosuppression and the preparation cycle of autologous CAR-T cells, we decided to prepare allogeneic CAR-T cells with donor-derived T cells, and arranged for our patient to receive CAR-T treatment as soon as possible. Subsequently, the patient developed CRS. After symptomatic treatment, her CRS symptoms were improved. CRS is a non-antigen specific toxicity and the most common toxicity reaction of CAR-T therapy, which is caused by the high-level CAR-T cells activation and target tumor cells lysis (12). The clinical manifestations of CRS are elevated serum inflammatory factors and uncontrollable high fever, similar to infection (13). However, CRS and infection have completely different the treatment strategy. Therefore, in order to use targeted drugs, it is necessary to identify CRS and infection. Undeniably, allogeneic CAR-T cells may cause severe GVHD, and may be quickly cleared away by the host immune system (11,14). This is something that deserves our a rapid attention. With the continuous development of new technologies, allogeneic CAR-T cell therapy is constantly optimized, becoming safer and more effective, and it may become the first-line treatment option for malignant tumor cases with poor prognosis and insufficient treatment approaches.
At present, CAR-T cell therapy has achieved favourable outcomes in B cell malignant tumors, such as acute B cell lymphoblastic leukemia and diffuse large B-cell lymphoma. However, due to differential expression of tumor cell antigen, the decrease of the number of CAR-T cells in vivo and the diversity of bone marrow microenvironment, patients still have a high risk of relapse after CAR-T therapy (15). Therefore, it is necessary to seek bridging therapy to prolong event-free survival rate (EFS) and relapse-free survival rate (RFS) of patients with high risk factors after CAR-T therapy. In general, HSCT is still considered as the best method to treat refractory/relapsed leukemia and lymphoma, which should be performed after patient reach complete remission (CR) (15,16). Some people even think that CAR-T cell therapy can be used as a part of conditioning regimen before HSCT. Genome-wide sequencing results showed that not only MYC gene was activated, but also mutations of TP53 gene and DDX3X gene were found in the patient. Previous studies have confirmed that these gene mutations are related to the poor prognosis in BL (17,18). Our patients had adverse molecular genetic changes, does not respond to multi-line chemotherapy, and has her identical donor, so she was bridged to allo-HSCT as soon as possible after CAR-T therapy. GVHD, as a major complication of HSCT, affects the immune disorders of organ systems (19). Organs affected by GVHD include gastrointestinal skin, tract, liver and lung. The treatment of GVHD requires long-term or even lifelong use of immunosuppressive drugs. At the same time, TA-TMA is the most difficult complication to identify and treat in transplant recipients (20). TA-TMA is characterized by microangiopathic hemolytic anemia and thrombocytopenia, which only affects arterioles and most often affects the kidney and gastrointestinal tract (21). According to the criteria proposed by Cho et al. (22), our patients were considered to have TA-TMA. Supportive treatment, including the elimination of calcineurin inhibitors and sirolimus, which may be the toxic drugs, appropriate antimicrobial therapy and renal replacement therapy such as CRRT, can alleviate or even reverse the patient’s condition to some extent (8). However, the patient developed sepsis while waiting for the implantation of white blood cells and platelet. Despite the active treatment for the first time, our patient still failed to survive.
In conclusion, we reported a rare case of refractory BLs, primarily located in the cervix. After allogeneic CAR-T therapy, CR was obtained, and the transition to allo-HSCT treatment was made. To our knowledge, this is the first report of allogeneic CAR-T cell therapy bridging to allo-HSCT in the treatment of primary BL of the cervix. Unfortunately, before leukocyte implantation, the patient developed TA-TMA and sepsis, and eventually died of septic shock. Nevertheless, we believe that donor-derived CAR-T cells could provide better treatment opportunities for patients with abnormal T cell function, and help patients with rapid disease progression to receive treatment as soon as possible. For patients with relapsed or refractory lymphoma, allogeneic CAR-T therapy is an effective therapeutic strategy for bridging to allo-HSCT. Undeniably, whether donor-derived CAR-T cell therapy will aggravate complications related to allo-HSCT need further study. This will be the focus of our future clinical work, and we expect to get a relatively clear result.
Acknowledgments
We owe thanks to the patient and her family.
Funding: This work was supported in part by the Natural Science Foundation of Hubei Province (No.2019CFB656 to LJ).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-174/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-174/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-174/coif). LJ received funding from the Natural Science Foundation of Hubei Province (No. 2019CFB656). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dunleavy K, Little RF, Wilson WH. Update on Burkitt Lymphoma. Hematol Oncol Clin North Am 2016;30:1333-43. [Crossref] [PubMed]
- Hilal Z, Hartmann F, Dogan A, et al. Lymphoma of the Cervix: Case Report and Review of the Literature. Anticancer Res 2016;36:4931-40. [Crossref] [PubMed]
- Casulo C, Friedberg J. Treating Burkitt Lymphoma in Adults. Curr Hematol Malig Rep 2015;10:266-71. [Crossref] [PubMed]
- Dunleavy K. Approach to the Diagnosis and Treatment of Adult Burkitt’s Lymphoma. J Oncol Pract 2018;14:665-71. [Crossref] [PubMed]
- Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 2020;135:17-27. [Crossref] [PubMed]
- Zhou X, Ge T, Li T, et al. CAR19/22 T cell therapy in adult refractory Burkitt’s lymphoma. Cancer Immunol Immunother 2021;70:2379-84. [Crossref] [PubMed]
- Liu J, Wang L, Yang H, et al. A narrative review of critical factors for better efficacy of CD19 chimeric antigen receptor T cell therapy in the treatment of B cell malignancies. Transl Cancer Res 2020;9:5655-62. [Crossref] [PubMed]
- Rosenthal J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med 2016;7:181-6. [Crossref] [PubMed]
- Dobrosavljevic A, Skrobic M, Stanojevic D, et al. Primary non-Hodgkin lymphoma of the uterine cervix of a follicular type – case report. J Obstet Gynaecol 2016;36:685-6. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15:31-46. [Crossref] [PubMed]
- Depil S, Duchateau P, Grupp SA, et al. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov 2020;19:185-99. [Crossref] [PubMed]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. [Crossref] [PubMed]
- Luo H, Wang N, Huang L, et al. Inflammatory signatures for quick diagnosis of life-threatening infection during the CAR T-cell therapy. J Immunother Cancer 2019;7:271. [Crossref] [PubMed]
- Kim DW, Cho JY. Recent Advances in Allogeneic CAR-T Cells. Biomolecules 2020;10:263. [Crossref] [PubMed]
- Jiang H, Hu Y, Mei H. Consolidative allogeneic hematopoietic stem cell transplantation after chimeric antigen receptor T-cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia: who? When? Why? Biomark Res 2020;8:66. [Crossref] [PubMed]
- Jiang H, Li C, Yin P, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol 2019;94:1113-22. [Crossref] [PubMed]
- Jiang L, Gu ZH, Yan ZX, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet 2015;47:1061-6. [Crossref] [PubMed]
- Greenough A, Dave SS. New clues to the molecular pathogenesis of Burkitt lymphoma revealed through next-generation sequencing. Curr Opin Hematol 2014;21:326-32. [Crossref] [PubMed]
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet 2009;373:1550-61. [Crossref] [PubMed]
- Khosla J, Yeh AC, Spitzer TR, et al. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant 2018;53:129-37. [Crossref] [PubMed]
- Yamada R, Nemoto T, Ohashi K, et al. Distribution of Transplantation-Associated Thrombotic Microangiopathy (TA-TMA) and Comparison between Renal TA-TMA and Intestinal TA-TMA: Autopsy Study. Biol Blood Marrow Transplant 2020;26:178-88. [Crossref] [PubMed]
- Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010;90:918-26. [Crossref] [PubMed]


