
Identification of hub genes associated with bladder cancer using bioinformatic analyses
Introduction
Bladder cancer (BLCA) is a major economic burden on society and the ninth most common cancer worldwide, with more than 500,000 new diagnoses and 200,000 deaths annually worldwide (1,2). The disease is more common in men, with a male-to-female ratio of 3:1, and disproportionately affects older adults, with a median age of 69 years for men and 71 years for women at diagnosis (3). Approximately 90–95% of BLCA cancers are urothelial cell carcinoma, while the remainder are non-urothelial tissue carcinoma (1,4). BLCA is associated with relapse and disease progression. The 5-year overall survival (OS) has been reported to be 90%, however, muscle invasive bladder cancer with high metastasis and a 5-year survival rate <50% (5). Unfortunately, there has been no significant progress in the treatment of BLCA in the last 30 years, and the disease is often diagnosed at an advanced stage (6). Thus, the diagnosis and treatment of BLCA requires improvement.
The combination of urine cytology and cystoscopy is the current gold standard for diagnosing BLCA (7). However, existing urine biomarkers are unreliable. Although cystoscopy is the most efficient and accurate diagnostic method, it is invasive and costly (8-10), and 5.5% of patients undergoing cystoscopy develop urinary tract infection (11). Therefore, there is an urgent need to discover new and reliable BLCA biomarkers.
The United States Food and Drug Administration (FDA) recently approved BTA stat (Polymedco), BTA TRAK (Polymedco), NMP22 enzyme-linked immunosorbent assay (Matritech), NMP22 BladderChek Test (Alere), uCyt (Scimedx), and UroVysion (Abbott Molecular) for use alongside cystoscopy for BLCA diagnosis and surveillance (12). However, the sensitivity and specificity of these reagents are lower than cystoscopy, and these biomarkers are typically used in combination with cystoscopy (11,13).
In this study, The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO) databases were searched for potential BLCA diagnostic and therapeutic targets. Ten genes were revealed as potential BLCA biomarkers, including kinesin family member 11 (KIF11), DLG associated protein 5 (DLGAP5), non-SMC condensin I complex subunit G (NCAPG), cell division cycle 20 (CDC20), cyclin B2 (CCNB2), BUB1 mitotic checkpoint serine (BUB1B), TPX2 microtubule nucleation factor (TPX2), NUF2 component of NDC80 kinetochore complex (NUF2), kinesin family member 2C (KIF2C), and cyclin B1 (CCNB1). These markers were associated with the prognosis of patients with BLCA, and 9 were associated with immunity, including KIF11, DLGAP5, NCAPG, CDC20, CCNB2, BUB1B, NUF2, KIF2C, and CCNB1. Four of these genes (BUB1B, CCNB1, CDC20, and DLGAP5) were identified as prognostic predictors and novel therapeutic targets for patients with BLCA.
We present the following article in accordance with the STREGA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1004/rc).
Methods
Microarray data
BLCA RNA sequence data and clinical information were downloaded from the GEO and TCGA databases. Hence, ethics committee approval or consent procedure was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The GSE147983 [GPL20301, Illumina HiSeq 4000 (Homo sapiens)] dataset containing 4 control and 4 BLCA tissue samples was obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The TCGA-BLCA mRNA dataset containing 19 normal and 414 tumor samples and related clinical data was downloaded from TCGA (https://portal.gdc.cancer.gov/).
Data processing
The online software GEO2R was used to analyze the differentially expressed genes (DEGs) in the GSE147983 dataset. The TCGA-BLCA mRNA dataset was processed using the “Limma” package in R version 4.0.4 (64 bit; The R Foundation for Statistical Computing, Vienna, Austria) (14). P<0.05 and Log|FC|≥2 were used as the cutoff criteria for BLCA-mRNA. Volcano maps were drawn using the “ggplot2” package in R, with log|FC|≥1 and P<0.05 as the screening criteria. The online tool Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to identify the overlapping DEGs in the 2 gene expression microarrays and determine upregulated and downregulated genes.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis
To better understand the role of DEGs in biological processes and signal transduction, the “clusterprofiler” R package was used to conduct GO and KEGG pathway analysis (15). P<0.05 was considered statistically significant.
Protein–protein interaction (PPI) network analysis
To further elucidate the molecular mechanisms of BLCA, the Search Tool for the Retrieval of Interacting Genes (STRING) online database (https://string-db.org/) was used to construct a DEG interaction network (16). The relationship between DEGs was then visualized using Cytoscape 3.7.2 software (17). The Cytoscape plugin app Molecular Complex Detection (“MCODE”) was also used to reanalyze the clusters in the network according to the following parameters: degree cutoff =2, node score cutoff =0.2, k-core =2, max.depth =100. The top 2 modules were selected, and the top 10 hub genes were screened using the Cytoscape plugin “cytoHubba”.
Gene expression analysis
The Gene Expression Profiling Interactive Analysis 2 (GEPIA2) (http://gepia2.cancer-pku.cn/#index) (18) database was used to visualize hub gene expression in BLCA and paracarcinoma tissues. P<0.05 was considered statistically significant.
Genomic alteration of the 10 hub genes using the cBioPortal database
The cBioPortal database (https://www.cbioportal.org/) was used to study the genomic mutations of the 10 hub genes in BLCA. Genomic alteration types and alteration frequency in BLCA were analyzed (19,20). The genomic alterations of the 10 hub genes contained missense/splice/truncating mutations with unknown significance, deep deletion, and amplification.
Immune infiltration in BLCA with different somatic copy number alterations (SCNAs)
SCNA analysis of the 10 hub genes was conducted using the Tumor Immune Estimation Resource (TIMER; https://cistrome.shinyapps.io/timer/) (21). The “SCNA” module was used to compare the tumor infiltration levels among tumors with different SCNAs for the 10 hub genes. In TIMER, SCNAs are categorized into 5 groups by the Genomic Identification of Significant Targets in Cancer (GISTIC) version 2.0, including deep deletion [−2], arm-level deletion [−1], diploid/normal [0], arm-level gain [1], and high amplification [2]. Box plots were drawn to show the distributions of each immune subset at each copy number status in selected cancers. The infiltration level for each SCNA category was compared with the normal control using a two-sided Wilcoxon rank-sum test.
Survival analysis of the hub genes
A survival analysis of the hub genes was performed using the online Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php?p=background) (22), which assesses the correlation between OS and different tumor genes.
Statistical analyses
The gene expression level and survival of BLCA were analyzed using GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS 22.0 (IBM Corp., Armonk, NY, USA). The data of the 2 groups were analyzed using a Student’s t-test. The results are presented as mean ± standard deviation (SD). P<0.05 was considered a statistically significant difference.
Results
Identification of DEGs
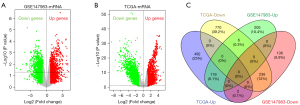
Based on the inclusion criteria, 1,403 and 4,597 DEGs were extracted from the GSE147983 and TCGA-BLCA mRNA datasets using the R “Limma” package and visualized using volcano plots, respectively (Figure 1A,1B). Overlapping DEGs were identified via a Venn diagram. Compared with the normal bladder tissues, 355 common DEGs were discovered in the BLCA tissues, including 236 upregulated genes (P<0.05, log2FC ≥2) and 119 downregulated genes (P<0.05, log2FC ≤−2; Figure 1C).
GO and KEGG pathway analyses of DEGs
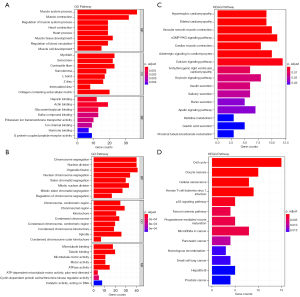
The R “ClusterProfiler” package was used to annotate and enrich the GO and KEGG pathways for the DEGs. The top 3 biological processes, cellular components, and molecule functions in the upregulated DEGs were muscle system process, regulation of muscle system process, and muscle contraction; myofibril, sarcomere, and contractile fiber; and heparin-binding, actin binding, and glycosaminoglycan binding, respectively. The top 3 biological processes, cellular components, and molecule functions in the downregulated DEGs were enriched in chromosome segregation, nuclear division, and organelle fission; chromosome—centromeric region, chromosomal region, and kinetochore; and microtubule binding, tubulin binding, and microtubule motor activity, respectively (Figure 2A,2B). The top 3 KEGG pathways of the upregulated DEGs were hypertrophic cardiomyopathy, dilated cardiomyopathy, and vascular smooth muscle contraction, and the top 3 KEGG pathways of the downregulated DEGs were cell cycle, oocyte meiosis, and cellular senescence (Figure 2C,2D).
PPI network analysis and hub genes screening
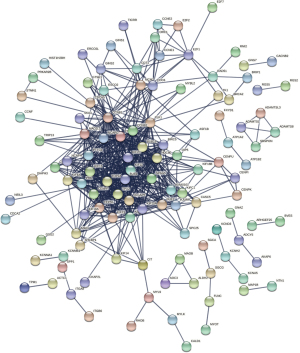
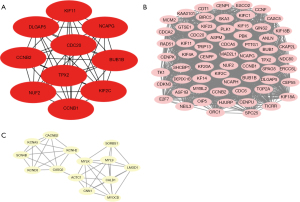
The 355 overlapping DEGs were imported into the STRING database for PPI network analysis (Figure 3), and the hub genes inside the network were screened using Cytoscape 3.7.2. The top 10 genes with the highest degree of connectivity were regarded as the hub genes (Figure 4A). To further explore the associations within the PPI network, the top 2 modules inside the PPI network were extracted using the “MCODE” package in Cytoscape (Figure 4B,4C).
Expression levels of the 10 hub genes in BLCA
To assess the expression levels of the hub genes in BLCA tissues, GEPIA2 was used to determine their expressions. The expression of BUB1B, CCNB1, CCNB2, CDC20, DLGAP5, KIF2C, KIF11, NCAPG, NUF2, and TPX2 was significantly increased in tumor tissues compared to that in the normal control, which was consistent with previous results (Figure 5).
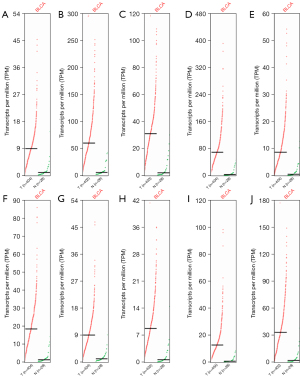
Genetic alteration analysis of the 10 hub genes
Genomic mutations are closely related to tumorigenesis. Hence, the genomic mutations of the top 10 hub genes in BLCA were analyzed. The results showed that approximately 2.1%, 2.3%, 1.3%, 4%, 1%, 3%, 7%, 13%, 2.8%, and 2.4% of genetic alterations were presents in KIF11, DLGAP5, NCAPG, CDC20, CCNB2, BUB1B, TPX2, NUF2, KIF2C, and CCNB1, respectively, in BLCA, including missense/splice/truncating mutations/structural variants with unknown significance, amplification, and deep deletion (Figure 6A). Moreover, the genetic alteration type and frequency of the 10 hub genes showed significant differences in BLCA (Figure 6B), indicating that the genetic alterations of the 10 hub genes could play an important role in the tumorigenesis of BLCA.

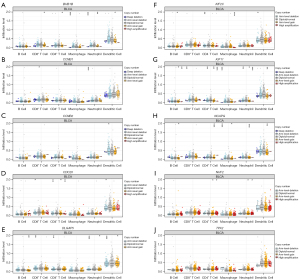
The association of the SCNAs of the 10 hub genes with immune infiltration
The importance of immune surveillance in determining the prognosis of various types of cancers is widely accepted. To further explore the relationship between the genomic metrics of the 10 hub genes and the extent of immune infiltration in BLCA, the SCNAs of the 10 hub genes were defined using GISTIC 2.0 in TIMER. The results showed that immune cell enrichment was significantly different in BLCA with different gene SCNAs (Figure 7). Furthermore, BLCA with the SCNA of BUB1B showed decreased cytotoxic T cell (CD8+ T), T helper cell (CD4+ T), macrophage, neutrophil, and dendritic cell enrichment (Figure 7A); CCNB1 decreased CD8+ T cell and neutrophil cell enrichment (Figure 7B); CCNB2 decreased dendritic cell enrichment (Figure 7C); CDC20 decreased CD8+ T cell, CD4+ T cell, and dendritic cell enrichment (Figure 7D); DLGAP5 decreased B cell, CD8+ T cell, CD4+ T cell, neutrophil, and dendritic cell enrichment (Figure 7E); KIF2C decreased B cell, CD4+ T cell, macrophage, and dendritic cell enrichment (Figure 7F); KIF11 decreased enrichment in all 6 immune cell types (Figure 7G); NCAPG decreased B cell, CD4+ T cell, and neutrophil cell enrichment (Figure 7H); and NUF2 decreased CD4+ T cell, neutrophil, and dendritic cell enrichment (Figure 7I). However, the immune cell enrichment showed no difference in BLCA with the SCNA of TPX2 (Figure 7J). Therefore, the genomic alterations of the 10 hub genes were strongly correlated with the extent of immune infiltration in BLCA.
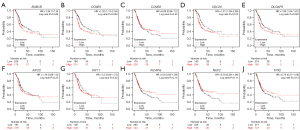
Kaplan-Meier analysis of the hub genes
The clinical significance of the hub genes was analyzed using the Kaplan-Meier Plotter online software. The results showed that patients with higher BUB1B, and CCNB1 expression levels had worse OS (P<0.05, Figure 8A,8B), while CCNB2 had better OS (P>0.05, Figure 8C). High CDC20, and DLGAP5 expression levels had worse OS (P<0.05, Figure 8D,8E), whereas a significant change was not observed in the rest genes (Figure 8F-8J).
Discussion
BLCA is the ninth most common cancer worldwide. Its incidence varies by region, with more than 60% of cases occurring in less developed countries (23). Approximately 75% of patients are diagnosed with non-muscle-invasive BLCA, while the remainder have already progressed to the muscle-invasive stage at diagnosis (24). Although new diagnostic and treatment strategies have recently been developed, only a minimal improvement in the clinical efficacy of these strategies has been reported (25). Therefore, identifying new diagnostic markers, therapeutic targets, and treatment methods remains crucial to the diagnosis and treatment of BCLA.
This study used the GSE147983 and TCGA-BLCA mRNA datasets from the GEO and TCGA databases to conduct a comparative analysis of the DEGs in BLCA tissues and normal controls. BUB1B, CCNB1, CCNB2, CDC20, DLGAP5, KIF2C, KIF11, NCAPG, NUF2, and TPX2 were found to be highly expressed in the BLCA tissues, suggesting their potential as diagnostic biomarkers for BLCA. Additionally, BUB1B, CCNB1, CCNB2, CDC20, DLGAP5, KIF2C, KIF11, NCAPG, and NUF2 were found to be associated with immune cell infiltration. The high expression of BUB1B, CCNB1, CDC20, and DLGAP5 was associated with worse OS in BLCA and could thus be considered an independent prognostic indicator for BLCA. These findings aid in identifying new diagnostic methods and treatment targets for BLCA, which could improve the prognosis of patients with BLCA.
BUB1B encodes a kinase involved in the spindle checkpoint function. Impaired spindle checkpoint function has been reported in many cancer types (26,27). Previous studies have reported that BUB1B is upregulated in BLCA (28,29), which is consistent with the results of this study. However, the immune role of BUB1B in BLCA remains undefined. Furthermore, BUB1B has been associated with immune infiltration in various tumors, such as prostate cancer, papillary renal cell carcinoma, and hepatocellular carcinoma (HCC) (30-32). In our immune infiltration analyses, BUB1B reduced CD8+ T cell, CD4+ T cell, macrophage, neutrophil, and dendritic cell enrichment, which validated the association of BUB1B with immune infiltration in BLCA.
CCNB1 and CCNB2 are important components of the cyclin pathway and play a key role in the occurrence and development of cancer (33). CCNB1 is involved in tumorigenesis and tumor development (34). Egloff et al. eluted CCNB1-derived peptides from major histocompatibility complex (MHC) class I molecules on tumor cells and revealed that this constitutively overexpressed protein was naturally processed into peptides that bind to MHC class I molecules and stimulate CD8+ T cells (35). In our study, CCNB1 decreased CD8+ T cell and neutrophil cell enrichment, confirming the association of CCNB1 with immune infiltration in BLCA. CCNB2 overexpression has been reported to be related to poor prognosis in HCC (36) and to promote invasion and metastasis in BLCA (37). Ni et al. found that CCNB2 could act as a biomarker and potential target for lung cancer treatment (38), while Xia et al. and Zou et al. reported the potential of CCNB2 as a prognostic biomarker and its association with immune cell infiltration in HCC and breast cancer (39,40). In our study, CCNB2 was highly expressed in BLCA and was associated with decreased dendritic cell enrichment, which suggested that CCNB2 could affect the immune process of BLCA.
Various studies have reported the association of CDC20 with the occurrence and development of different tumors, including BLCA (41). In most cancer types, CDC20 expression is positively correlated with the infiltration of cancer-associated fibroblasts and myeloid-derived suppressor cells (41). Our analysis of the relationship between CDC20 and tumor immunity revealed that CDC20 decreased CD8+ T cell, CD4+ T cell, and dendritic cell enrichment in BLCA.
DLGAP5 protein, also known as hepatoma upregulated protein (HURP) or KIAA0008, was first identified as a cell cycle–regulated protein (42). Various studies have focused on the role of DLGAP5 in the tumorigenesis of liver cancer (43), pancreatic cancer (44), lung cancer (45), and ovarian cancer (46). However, its role in BLCA remains unexplored. In this study, DLGAP5 was highly expressed in BLCA, and the correlation between DLGAP5 and B cell, CD8+ T cell, CD4+ T cell, neutrophil, and dendritic cell enrichment was demonstrated.
KIF2C and KIF11 belong to the kinesin family. The functions of KIF2C are related to the microtubule-dependent molecular motor and chromosome positioning processes, while the functions of KIF11 are related to the centrosome separation and bipolar spindle establishment during cell mitosis processes (29). Their abnormal expression has been associated with the prognosis of BLCA (47,48). Additionally, KIF2C and KIF11 have been reported to affect the immune microenvironment in some tumors (49,50), but their role in BLCA requires further elucidation. In the present study, KIF2C and KIF11 were found to be associated with immune cell enrichment.
Reports on the role of NUF2 and NCAPG in BLCA are scarce. This study revealed that NUF2 and NCAPG play a critical role in BLCA diagnosis. Functional analysis showed that NUF2 was associated with CD4+ T cell, neutrophil, and dendritic cell enrichment in BLCA, while NCAPG was related to B cell, CD4+ T cell, and neutrophil cell enrichment.
Previous trials have indicated that TPX2 is associated with the metastasis and prognosis of BLCA (51,52). However, the present study observed that although TPX2 was upregulated in BLCA tissues, higher expression levels were associated with better OS. Further validation of the expression status of TPX2 revealed that TPX2 expression was upregulated in BLCA. To date, only a few studies have reported the exact biological role of TPX2 (51,52), and its potential mechanisms in the diagnosis, progression, and immunity of BLCA remain unclear.
Despite its advantages, this study has certain limitations, including the lack of in vivo and in vitro validations. Furthermore, although 10 hub genes were found to be upregulated in BLCA, the mechanism of upregulation was unclear. Therefore, further molecular studies are needed to determine the function of these central genes and their role in the progression of BLCA.
Conclusions
This study identified 10 potential biomarkers of BLCA, including KIF11, DLGAP5, NCAPG, CDC20, CCNB2, BUB1B, TPX2, NUF2, KIF2C, and CCNB1, which could be used as diagnostic indicators of BLCA. Nine of these genes were associated with immunity, including KIF11, DLGAP5, NCAPG, CDC20, CCNB2, BUB1B, NUF2, KIF2C, and CCNB1. Additionally, 4 of these genes (BUB1B, CCNB1, CDC20, and DLGAP5) have the potential to be prognostic predictors and novel therapeutic targets for BLCA. However, further studies are required to validate these findings. Thus, this study provides a strong basis for the development of BLCA gene-targeted therapies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1004/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1004/coif). XZ is from CheerLand Clinical Laboratory Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lenis AT, Lec PM, Chamie K, et al. Bladder Cancer: A Review. JAMA 2020;324:1980-91. [Crossref] [PubMed]
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin 2020;70:404-23. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J 2009;3:S193-8. [Crossref] [PubMed]
- Wang Y, Chen L, Ju L, et al. Novel Biomarkers Associated With Progression and Prognosis of Bladder Cancer Identified by Co-expression Analysis. Front Oncol 2019;9:1030. [Crossref] [PubMed]
- Grayson M. Bladder cancer. Nature 2017;551:S33. [Crossref] [PubMed]
- Sun M, Trinh QD. Diagnosis and staging of bladder cancer. Hematol Oncol Clin North Am 2015;29:205-18. vii. [Crossref] [PubMed]
- Farling KB. Bladder cancer: Risk factors, diagnosis, and management. Nurse Pract 2017;42:26-33. [Crossref] [PubMed]
- Bladder cancer: diagnosis and management of bladder cancer: © NICE (2015) Bladder cancer: diagnosis and management of bladder cancer. BJU Int 2017;120:755-65. [Crossref] [PubMed]
- Ulamec M, Murgić J, Novosel L, et al. New Insights into the Diagnosis, Molecular Taxonomy, and Treatment of Bladder Cancer. Acta Med Acad 2021;50:143-56. [Crossref] [PubMed]
- Tan WS, Tan WP, Tan MY, et al. Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treat Rev 2018;69:39-52. [Crossref] [PubMed]
- Sugeeta SS, Sharma A, Ng K, et al. Biomarkers in Bladder Cancer Surveillance. Front Surg 2021;8:735868. [Crossref] [PubMed]
- Chou R, Gore JL, Buckley D, et al. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:922-31. [Crossref] [PubMed]
- Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [Crossref] [PubMed]
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. [Crossref] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49:D605-D612. Erratum in: Nucleic Acids Res 2021;49:10800. [Crossref] [PubMed]
- Doncheva NT, Morris JH, Gorodkin J, et al. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J Proteome Res 2019;18:623-32. [Crossref] [PubMed]
- Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 2019;47:W556-60. [Crossref] [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [Crossref] [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [Crossref] [PubMed]
- Li T, Fan J, Wang B, et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res 2017;77:e108-10. [Crossref] [PubMed]
- Wu W, Jia G, Chen L, et al. Analysis of the Expression and Prognostic Value of Annexin Family Proteins in Bladder Cancer. Front Genet 2021;12:731625. [Crossref] [PubMed]
- D'souza AA, Tulpule V, Zang PD, et al. Bladder cancer: from a therapeutic wilderness to so many options; a guide to practice in a changing landscape. Ann Oncol 2022;33:242-3. [Crossref] [PubMed]
- Dobruch J, Oszczudłowski M. Bladder Cancer: Current Challenges and Future Directions. Medicina (Kaunas) 2021;57:749. [Crossref] [PubMed]
- Liu S, Chen X, Lin T. Lymphatic metastasis of bladder cancer: Molecular mechanisms, diagnosis and targeted therapy. Cancer Lett 2021;505:13-23. [Crossref] [PubMed]
- Pinto M, Vieira J, Ribeiro FR, et al. Overexpression of the mitotic checkpoint genes BUB1 and BUBR1 is associated with genomic complexity in clear cell kidney carcinomas. Cell Oncol 2008;30:389-95. [PubMed]
- Myrie KA, Percy MJ, Azim JN, et al. Mutation and expression analysis of human BUB1 and BUB1B in aneuploid breast cancer cell lines. Cancer Lett 2000;152:193-9. [Crossref] [PubMed]
- Komura K, Inamoto T, Tsujino T, et al. Increased BUB1B/BUBR1 expression contributes to aberrant DNA repair activity leading to resistance to DNA-damaging agents. Oncogene 2021;40:6210-22. [Crossref] [PubMed]
- Pan S, Zhan Y, Chen X, et al. Identification of Biomarkers for Controlling Cancer Stem Cell Characteristics in Bladder Cancer by Network Analysis of Transcriptome Data Stemness Indices. Front Oncol 2019;9:613. [Crossref] [PubMed]
- Zhao HB, Zeng YR, Han ZD, et al. Novel immune-related signature for risk stratification and prognosis in prostatic adenocarcinoma. Cancer Sci 2021;112:4365-76. [Crossref] [PubMed]
- Huang R, Liu J, Li H, et al. Identification of Hub Genes and Their Correlation With Immune Infiltration Cells in Hepatocellular Carcinoma Based on GEO and TCGA Databases. Front Genet 2021;12:647353. [Crossref] [PubMed]
- Deng R, Li J, Zhao H, et al. Identification of potential biomarkers associated with immune infiltration in papillary renal cell carcinoma. J Clin Lab Anal 2021;35:e24022. [Crossref] [PubMed]
- Wang D, Sun H, Li X, et al. CCNB2 is a novel prognostic factor and a potential therapeutic target in low-grade glioma. Biosci Rep 2022;42:BSR20211939. [Crossref] [PubMed]
- Ye C, Wang J, Wu P, et al. Prognostic role of cyclin B1 in solid tumors: a meta-analysis. Oncotarget 2017;8:2224-32. [Crossref] [PubMed]
- Egloff AM, Vella LA, Finn OJ. Cyclin B1 and other cyclins as tumor antigens in immunosurveillance and immunotherapy of cancer. Cancer Res 2006;66:6-9. [Crossref] [PubMed]
- Li R, Jiang X, Zhang Y, et al. Cyclin B2 Overexpression in Human Hepatocellular Carcinoma is Associated with Poor Prognosis. Arch Med Res 2019;50:10-7. [Crossref] [PubMed]
- Lei CY, Wang W, Zhu YT, et al. The decrease of cyclin B2 expression inhibits invasion and metastasis of bladder cancer. Urol Oncol 2016;34:237.e1-10. [Crossref] [PubMed]
- Ni KW, Sun GZ. The identification of key biomarkers in patients with lung adenocarcinoma based on bioinformatics. Math Biosci Eng 2019;16:7671-87. [Crossref] [PubMed]
- Xia T, Meng L, Zhao Z, et al. Bioinformatics prediction and experimental verification identify MAD2L1 and CCNB2 as diagnostic biomarkers of rhabdomyosarcoma. Cancer Cell Int 2021;21:634. [Crossref] [PubMed]
- Zou Y, Ruan S, Jin L, et al. CDK1, CCNB1, and CCNB2 are Prognostic Biomarkers and Correlated with Immune Infiltration in Hepatocellular Carcinoma. Med Sci Monit 2020;26:e925289. [Crossref] [PubMed]
- Wu F, Sun Y, Chen J, et al. The Oncogenic Role of APC/C Activator Protein Cdc20 by an Integrated Pan-Cancer Analysis in Human Tumors. Front Oncol 2021;11:721797. [Crossref] [PubMed]
- Bassal S, Nomura N, Venter D, et al. Characterization of a novel human cell-cycle-regulated homologue of Drosophila dlg1. Genomics 2001;77:5-7. [Crossref] [PubMed]
- Kuo TC, Chang PY, Huang SF, et al. Knockdown of HURP inhibits the proliferation of hepacellular carcinoma cells via downregulation of gankyrin and accumulation of p53. Biochem Pharmacol 2012;83:758-68. [Crossref] [PubMed]
- Ke MJ, Ji LD, Li YX. Bioinformatics analysis combined with experiments to explore potential prognostic factors for pancreatic cancer. Cancer Cell Int 2020;20:382. [Crossref] [PubMed]
- Tagal V, Wei S, Zhang W, et al. SMARCA4-inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX-680 in non-small cell lung cancers. Nat Commun 2017;8:14098. [Crossref] [PubMed]
- Chen X, Thiaville MM, Chen L, et al. Defining NOTCH3 target genes in ovarian cancer. Cancer Res 2012;72:2294-303. [Crossref] [PubMed]
- Mo XC, Zhang ZT, Song MJ, et al. Screening and identification of hub genes in bladder cancer by bioinformatics analysis and KIF11 is a potential prognostic biomarker. Oncol Lett 2021;21:205. [Crossref] [PubMed]
- Yang C, Li Q, Chen X, et al. Circular RNA circRGNEF promotes bladder cancer progression via miR-548/KIF2C axis regulation. Aging (Albany NY) 2020;12:6865-79. [Crossref] [PubMed]
- Li Z, Yu B, Qi F, et al. KIF11 Serves as an Independent Prognostic Factor and Therapeutic Target for Patients With Lung Adenocarcinoma. Front Oncol 2021;11:670218. [Crossref] [PubMed]
- An L, Zhang J, Feng D, et al. KIF2C Is a Novel Prognostic Biomarker and Correlated with Immune Infiltration in Endometrial Cancer. Stem Cells Int 2021;2021:1434856. [Crossref] [PubMed]
- Li F, Su M, Zhao H, et al. HnRNP-F promotes cell proliferation by regulating TPX2 in bladder cancer. Am J Transl Res 2019;11:7035-48. [PubMed]
- Yan L, Li Q, Yang J, et al. TPX2-p53-GLIPR1 regulatory circuitry in cell proliferation, invasion, and tumor growth of bladder cancer. J Cell Biochem 2018;119:1791-803. [Crossref] [PubMed]
(English Language Editor: C. Gourlay)


