
Hemophagocytic lymphohistiocytosis in two patients following treatment with pembrolizumab: two case reports and a literature review
Introduction
Immune checkpoint inhibitors (ICIs) provide an innovative and effective alternative for tumor therapy. They activate the immune system to produce antitumor immune responses, but overactivation of the immune system may cause immune-related adverse events (irAEs) ranging from mild rash to severe colitis or fatal myocarditis (1). Recently, hemophagocytic lymphohistiocytosis (HLH), a rare and life-threatening state of immune hyperactivation with no exact pathogenesis, has also been reported to be triggered by ICIs during treatment of various cancers (2). However, factors that predispose a patient to HLH, such as underlying malignancies, concomitant infections, or autoimmune diseases (ADs), are rarely mentioned in literature about ICIs. Furthermore, the diagnosis of HLH may be delayed since it is currently based on the HLH-2004 diagnostic criteria, which was developed for children and lacks specific markers (3). Useful indicators are needed to predict the early emergence of HLH.
Here, we report 2 cases of HLH following treatment with pembrolizumab, both of which were successfully treated with dexamethasone and etoposide. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-154/rc).
Case presentation
Patient 1
A 50-year-old woman was diagnosed with thymic carcinoma (TC) (cT1aN2M1a stage IVB). Dry-mouth symptoms and positive anti-SS-A and anti-SS-B antibodies indicated a possible diagnosis of Sjögren’s syndrome (SS), but the patient refused further examination. A cycle of carboplatin plus paclitaxel was implemented as a first-line treatment, following which, the patient experienced digestive toxicity and grade III myelosuppression. The treatment was changed to a second-line option involving immunotherapy with 200 mg of pembrolizumab.
At 7 days after pembrolizumab administration, the patient developed a moderate fever and aggravated dry mouth. Chest computed tomography (CT) showed progressive interstitial inflammation and splenomegaly, accompanied by smaller primitive neoplasms and metastases (Figure 1). With no signs of infection, SS was suspected, so 8 mg of oral methylprednisolone (once a day) and 50 mg of azathioprine (twice a day) were prescribed. The patient rapidly defervesced, but the symptom of dry mouth was not relieved.
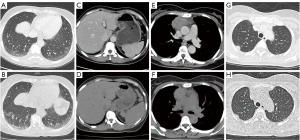
The patient developed a recurrent high-grade fever on day 18 after immunotherapy, after the accumulated use of 40 mg of methylprednisolone. Oral methylprednisolone and azathioprine were withdrawn. The patient had elevated liver enzymes (Figure 2), thrombocytopenia (59,000/µL), hypertriglyceridemia (3.17 mmol/L), hypofibrinogenemia (0.37 g/L), and increased ferritin (2,000 µg/L), but screening tests for bacteria and fungi were negative. Out of the 8 diagnostic criteria for HLH described in HLH-2004, 4 were met, including a hemoglobin level of 91 g/L on day 24, thus treatment with dexamethasone and etoposide was initiated (3). The hemoglobin level decreased to 84 g/L the next day, and additional workup revealed a decreased natural killer (NK) cell function and elevated soluble CD25 (sCD25) (142,225 pg/mL). Eventually, this case met 7 out of the 8 diagnostic criteria for HLH (4).
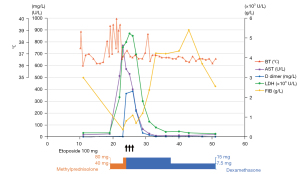
The patient responded well to the therapy; although the severe cytopenia persisted, which was suspected to be attributed to etoposide. Eventually, she was safely discharged, the mediastinal mass and metastases was markedly diminished, and HLH was successfully controlled without relapse in 10 months.
Patient 2
A 70-year-old man was diagnosed with squamous cell carcinoma of the lung (cT3N2M1c staged IVB). His antinuclear antibody was positive, and he had no history of AD. He received an initial cycle of chemotherapy with carboplatin plus paclitaxel. Subsequently, both his lower and left upper extremities presented pitting edema, which was suspected to be related to hypoalbuminemia. A second cycle of chemotherapy was administrated along with 100 mg of pembrolizumab for immunotherapy.
After 7 days, the patient developed a fever of 38 ℃ with pain and edema in both lower limbs but defervesced the next day after the initiation of antibiotics. Additionally, ultrasonography of the abdomen showed a slightly enlarged spleen on day 10.
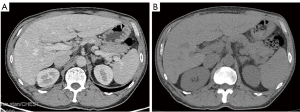
On day 12, after pembrolizumab administration, the patient presented with a repeated fever, which had reached up to 40.5 ℃. Corticotherapy of 40 mg of methylprednisolone was initiated to treat suspected irAEs. However, the patient’s liver function deteriorated rapidly and he had a level of serum glutamic oxaloacetic transaminase of 2,378.3 U/L. The CT results showed splenomegaly (Figure 3). Highly elevated ferritin (>2,000 µg/L) and the quantitative determination of the Epstein-Barr virus (EBV) DNA (20,600 copies/mL) led to a suspected diagnosis of HLH, after which, corticotherapy was intensified with 10 mg/m2 of dexamethasone. Subsequently, testing revealed elevated sCD25 (26,673 pg/mL) and hypertriglyceridemia (2.46 mmol/L), and bone marrow aspiration showed hemophagocytic macrophages. The patient satisfied the clinical diagnostic criteria of HLH (5 out of 8) on day 24. Therefore, etoposide (100 mg) was added for 3 days. Soon, HLH was controlled, without relapse in 8 months.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Characteristically, HLH involves the uncontrolled activation of cytotoxic lymphocytes and macrophages, resulting in cytokine-mediated tissue injury and multiorgan dysfunction (2). The associated mortality rate has been estimated at 23%, the highest among all hematological irAEs; but the mechanism of how HLH is triggered by ICIs is not well understood (2,5). Early diagnosis and treatment are critical to the prognosis of patients with HLH (2). The 2 cases we have reported may assist the determination of predisposing factors and increase the awareness of early diagnostics and therapies for HLH.
The administration of ICIs in patients with thymic epithelial tumors (TET) has shown promising activity, although with higher rates of immune toxicity than other cancers, which can be attributed to the intrinsic characteristics of these malignancies; but TC has shown higher response rates with lower rates of irAEs compared to thymomas (6,7). Furthermore, pembrolizumab has recently been added as a second-line option for the treatment of TC by the National Cancer Comprehensive Network (NCCN) guidelines considering the aggressive nature and the lack of effective treatment of TC (8).
Existing retrospective studies have shown that ICIs are safe to use in patients with preexisting ADs or positive antibodies under close monitoring (9,10). Associated irAEs and AD flares occur more frequently but are mostly mild and manageable without discontinuing ICIs treatment (9,10). However, no existing studies have evaluated the risk of ICI administration in TC patients with preexisting ADs. A phase II trial of pembrolizumab enrolled 33 TET patients, among whom 3 patients with preexisting AD developed severe irAEs, which led to the study protocol being revised to exclude patients with previous AD (11). Currently, there has only been 1 reported HLH case of a TC patient that was triggered by ICIs, but the patient had a history of psoriasis, which was under control, and died of HLH (12). The first patient documented in our report, who had a previous history of SS, developed severe HLH and nearly died as a result. The obvious predisposition to severe irAEs seems to exist in TC patients with ADs, even when these ADs are under control.
The second HLH case we reported was triggered by pembrolizumab when the patient had an EBV infection and positive antinuclear antibodies. An EBV infection is the most identified infectious trigger for HLH (2). The largest report in a meta-analysis of hematologic complications of ICIs recruited 26 HLH cases, and concomitant EBV infection was reported in 19% of the cases (13). Furthermore, HLH cases have been reported to occur in patients with an EBV infection or bacterial infection, in which the use of ICIs may produce a stronger immune response (14,15). These factors indicate that preexisting antibodies may be another factor that predisposes the patient to developing HLH.
Noseda et al. (16) analyzed 38 HLH cases in patients treated with ICIs in the World Health Organization (WHO) global database and showed that most reports did not mention underlying malignancies nor concomitant infections as contributors to HLH. Dupré et al. (17) described 20 HLH cases induced by ICIs and suggested that an infectious disease or the progression of cancer itself may be other associated causes of HLH. However, infection status, such as EBV status, was often not described in case reports. Although the exact pathogenesis of HLH is unknown, HLH is related to hyperinflammation, and Brisse et al. (18) portrayed it as a ‘threshold’ disease in which inflammation is weighted on different predisposing factors, such as genetic factors, infections, autoimmune disorders, and underlying malignancies, until a threshold is exceeded. Both cases we have reported had 2 predisposing factors that placed them at a high risk for HLH. Furthermore, TC patients with ADs are prone to severe irAEs. Since the infusion of ICIs may also be a predisposing factor for HLH, extra caution is required before the infusion of ICIs in patients with 2 or more predisposing factors, and screening tests for ADs and infections are recommended before the infusion of immunotherapy. An interesting phenomenon is that both cases we reported and the previously reported TC patient with psoriasis developed HLH after the first infusion of immunotherapy (12). The same occurred in the 3 TET patients with preexisting AD, who developed severe irAEs in a phase II trial of pembrolizumab (11). It seems that HLH, as well as other severe irAEs, often occurred after the first infusion of immunotherapy in patients with 2 or more predisposing factors. More research is needed to further examine this relationship.
Both patients experienced fever on day 7 and splenomegaly on day 10. A transient fever was also reported in other cases in existing literature (19,20). The concept of an immune-related fever that occurred 2–10 days after the infusion of ICIs, put forward by Michot et al. (21), may explain this phenomenon. The fever and splenomegaly may be signs of enhanced immune activation. Previous research has shown that diseases that present prior to the diagnosis of HLH, such as thyroiditis, appendicitis, edema, and flare-up of preexisting ADs, may be the expression of immune overactivation (19,22-24). Moreover, markedly elevated interleukin (IL)-6 was detected in both patients, which could be a possible treatment target for HLH. Tocilizumab (TCZ), an anti-IL-6R antibody, has been used to treat HLH and has shown rapid resolution of cytokine release syndrome and HLH (3,25). Considering the rarity and the high mortality rate of HLH, the immune-related fever and hyperinflammatory response may indicate the presence of HLH; however, further research is needed to confirm this relationship.
In conclusion, we have reported the cases of 2 patients who developed HLH following treatment with pembrolizumab. Preexisting ADs or positive antibodies, concomitant infection, and TET may be predisposing factors for HLH. Although HLH is rare, it has a high mortality rate. Given the widespread use of ICIs, extra caution is needed before the initiation of ICIs for patients with 2 or more predisposing factors. Immune-related fever, splenomegaly, and other signs of hyperinflammation may be indicators of the presence of HLH.
Acknowledgments
We would like to thank AME experts for their language editing services.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81770031, 81970031), the Natural Science Foundation of Jiangsu Province (No. BK20181497), the Key Research and Development Project of Jiangsu Province (No. BE2020616), and the General Projects of Jiangsu Province Commission of Health (No. M2020069).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-154/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-154/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-154/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol 2020;34:101515. [Crossref] [PubMed]
- La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133:2465-77. [Crossref] [PubMed]
- Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124-31. [Crossref] [PubMed]
- Zhuang J, Du J, Guo X, et al. Clinical diagnosis and treatment recommendations for immune checkpoint inhibitor-related hematological adverse events. Thorac Cancer 2020;11:799-804. [Crossref] [PubMed]
- Tateo V, Manuzzi L, De Giglio A, et al. Immunobiology of Thymic Epithelial Tumors: Implications for Immunotherapy with Immune Checkpoint Inhibitors. Int J Mol Sci 2020;21:9056. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Thymomas and Thymic Carcinomas. NCCN Guidelines 2021. Version 1.
- Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 2020;31:724-44. [Crossref] [PubMed]
- Fillon M. Immune checkpoint inhibitors may be safe for patients with preexisting autoimmune disease. CA Cancer J Clin 2020;70:3-4. [Crossref] [PubMed]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. [Crossref] [PubMed]
- Laderian B, Koehn K, Holman C, et al. Association of Hemophagocytic Lymphohistiocytosis and Programmed Death 1 Checkpoint Inhibitors. J Thorac Oncol 2019;14:e77-8. [Crossref] [PubMed]
- Davis EJ, Salem JE, Young A, et al. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 2019;24:584-8. [Crossref] [PubMed]
- Shah D, Shrestha R, Ramlal R, et al. Pembrolizumab associated hemophagocytic lymphohistiocytosis. Ann Oncol 2017;28:1403. [Crossref] [PubMed]
- Malissen N, Lacotte J, Du-Thanh A, et al. Macrophage activation syndrome: A new complication of checkpoint inhibitors. Eur J Cancer 2017;77:88-9. [Crossref] [PubMed]
- Noseda R, Bertoli R, Müller L, et al. Haemophagocytic lymphohistiocytosis in patients treated with immune checkpoint inhibitors: analysis of WHO global database of individual case safety reports. J Immunother Cancer 2019;7:117. [Crossref] [PubMed]
- Dupré A, Michot JM, Schoeffler A, et al. Haemophagocytic lymphohistiocytosis associated with immune checkpoint inhibitors: a descriptive case study and literature review. Br J Haematol 2020;189:985-92. [Crossref] [PubMed]
- Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol 2016;174:203-17. [Crossref] [PubMed]
- Kurozumi A, Takahashi H, Watanabe T, et al. Two cases of lung cancer with hemophagocytic lymphohistiocytosis caused by immune checkpoint inhibitors. Thorac Cancer 2021;12:1625-8. [Crossref] [PubMed]
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: A case report and literature review. Dermatol Ther 2020;33:e13321. [PubMed]
- Michot JM, Pruvost R, Mateus C, et al. Fever reaction and haemophagocytic syndrome induced by immune checkpoint inhibitors. Ann Oncol 2018;29:518-20. [Crossref] [PubMed]
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer 2018;93:150-3. [Crossref] [PubMed]
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer 2019;115:84-7. [Crossref] [PubMed]
- Akagi Y, Awano N, Inomata M, et al. Hemophagocytic Lymphohistiocytosis in a Patient with Rheumatoid Arthritis on Pembrolizumab for Lung Adenocarcinoma. Intern Med 2020;59:1075-80. [Crossref] [PubMed]
- Özdemir BC, Latifyan S, Perreau M, et al. Cytokine-directed therapy with tocilizumab for immune checkpoint inhibitor-related hemophagocytic lymphohistiocytosis. Ann Oncol 2020;31:1775-8. [Crossref] [PubMed]
(English Language Editors: C. Mullens and J. Jones)


