
Hyponatremia in small cell lung cancer is associated with a poorer prognosis
Introduction
Small cell lung cancer (SCLC) occurs in 15–20% of lung cancer. More than 60–70% of SCLC patients were with metastasis at diagnosis. New therapeutic method has not emerged currently and with median survival time under 1 year in extensive disease and no more than 2 years in limited disease SCLC (1,2).
Hyponatremia with SCLC patients occurs in approximately 15% (3). Hyponatremia is usually caused by the syndrome of inappropriate antidiuretic hormone (SIADH) or increased release of atrial natriuretic peptide which is one of the most frequent abnormalities of clinical laboratory tests. Other possible causes of hyponatremia at least in elderly patients with SCLC are chronic heart failure, nephritic syndrome and volume depletion (4). Symptoms of hyponatremia include nausea, fatigue, disorientation, muscle cramps and so on. Severe hyponatremia could lead to seizure, even to death (5).
At present, the prognostic significance of hyponatremia has been reported in several studies with a controversial conclusion. Several analyses illustrated results with some evidence for low sodium levels being a negative prognostic factor (6-11). While, other studies could not demonstrate the similar results (12,13).The inconclusive results may contribute to the small sample in previous studies.
This study focused on patients with hyponatremia and evaluated its prognostic value in SCLC.
Methods
Study population
We retrospectively evaluated patients with diagnosed SCLC from July 2006 to September 2013 in Zhejiang cancer hospital. SCLC was confirmed by pathologic histology in all cases.
We collected data on gender, age, disease stage, initial diagnostic date, performance status, serum sodium values, serum level of lactate dehydrogenase and hemoglobin, metastatic sites, chemotherapy regimens. And blood routine, biochemical and electrolyte were examined at regular intervals.
Hyponatremia was defined as serum sodium values below 135 mmol/L, and every patient was examined twice in the same laboratory instrument. Severe hyponatremia was present if sodium value was measured equal or below 129 mmol/L.
Statistical analysis
OS was defined as the time between the first day of therapy and death, whatever the cause. Observation time of patients alive without disease progression at the last follow-up visit was censored. The method of Kaplan and Meier was used to calculate survival curves. To assess the statistical significance of differences between the crude survival curves, we performed log-rank tests. Multivariate testing was done by Cox regression analyses. Analyses were conducted using the computer software SPSS version 19.0. The P value of less than 0.05 was regarded as statistically significant.
Results
Patient profile
In 631 SCLC patients, 241 patients (38.2%) had limited disease (LD) at time of diagnosis. And extensive disease (ED) SCLC was present in 390 (61.8%). The pre-treatment serum sodium values were available in all patients. Median sodium value was 136 mmol/L (range, 111–145 mmol/L). 103 patients (16.3%) were found serum sodium values below 135 mmol/L at pre-treatment or during treatment. Hyponatremia was present in 60 out of 390 patients with ED SCLC and 43 out of 241 patients with LD SCLC. We then divided the cases with low sodium levels in two groups (Na 130–134 mmol/L and Na ≤129 mmol/L). Mild hyponatremia (130–134 mmol/L) was detected in 52 out of 103 patients. Severe hyponatremia (sodium value at or below 129 mmol/L) was documented in the remaining 51 patients.
In prior treatment, there were 66 (66/103, 64.1%) hyponatremia patients. During treatment (at least after one cycle of chemotherapy), 37 patients happened hyponatremia. In the group of 66 patients, after at least two cycles of chemotherapy and additionally given sodium supplementation, 42 cases returned to normal; but the values remained subnormal sodium values were 24 patients. In the group of 37 patients who received sodium supplementation (for the lack of sodium, they were received sodium supplementation according to formula, and for dilutional hyponatremia, it included fluid restriction, diuresis and supplement hypertonic sodium), 10 patients returned to normal and 27 patients remained hyponatremia.
The basic characteristics of the all patients and hyponatremia patients are shown in Tables 1,2, respectively.

Full table
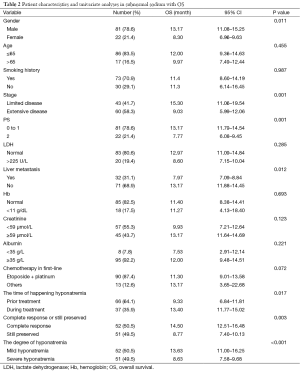
Full table
First-line therapy was performed with platinum-based regimens in all patients. In LD SCLC patients, thoracic radiotherapy (TRT) was employed in 79% and prophylactic cranial irradiation (PCI) was in 34% LD SCLC patients. In ED patients, 18% (69/390) patients with brain metastasis, 88% (61/69) received whole brain radiotherapy among them. And PCI was employed in 19% (63/321) remained ED patients.
Survival analysis
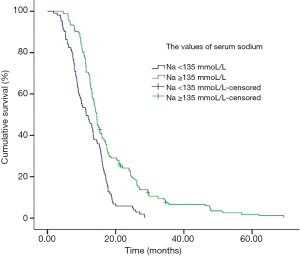
Median survival in the normal group of patients was significantly longer than patients with subnormal group (Table 3, Figure 1) (14.5 vs. 11.4 months, P<0.001). In subset analysis of patients with LD or ED SCLC, there was a shorter median survival in patients with subnormal sodium values than patients with normal group (Table 3).

Full table
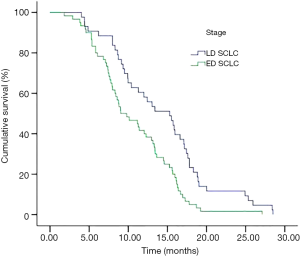
In subnormal sodium group, the median survival in LD and ED were 15.30 and 9.03 months respectively (P=0.001) (Figure 2). The OS of 66 patients who appeared pre-treatment hyponatremia was shorter than the remaining 37 patients happened during treatment (median survival was 9.33 vs. 13.40, P=0.017).
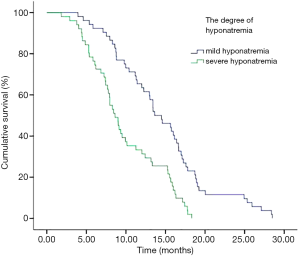
In addition, we performed analyses with regard to the degree of plasma sodium values. There were significant differences in OS between mild hyponatremia and severe hyponatremia (13.63 vs. 8.63, P<0.001) (Figure 3). Among 66 patients who appeared hyponatremia before treatment, the OS of severe hyponatremia had poorer survivals than mild hyponatremia (9.03 vs. 12.97 months, P=0.019) (Figure 4). The group of 37 patients that hyponatremia was present during treatment had the similar result (4.40 vs. 13.64 months, P<0.001) (Figure 5). On the other hand, patients who still remained hyponatremia with adequate treatment were prone to have shorter median survival than the group of patients with complete response (8.70 vs. 14.55 months, P=0.003) (Figure 6).
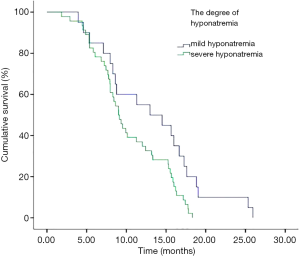
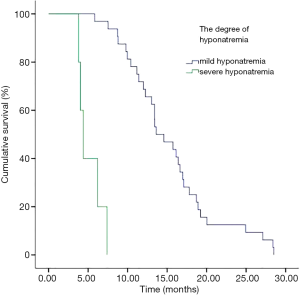
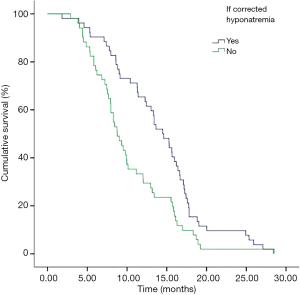
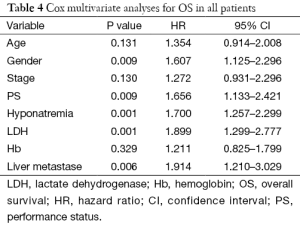
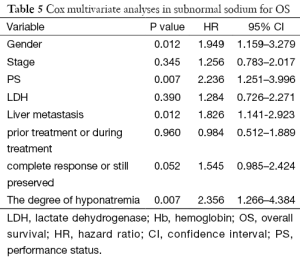
In a Cox multivariate analysis of the 631 patients, hyponatremia was associated with poorer prognosis (P<0.001, 95% CI, 1.34–2.47, HR=1.82) (Table 4). Univariate analyses were performed by Kaplan-Meier method to assess the predictive capability of each variable assessed in the group of hyponatremia with OS. These data are summarized in Table 2. Gender, PS, stage, liver metastasis, the degree of hyponatremia and the time of hyponatremia were predictive of overall survival (OS). In multivariate Cox regression analyses, the degree of hyponatremia was an independent predictor of mortality (P=0.007, HR=2.356). Other independent prognostic factors included gender, PS, liver metastasis (Table 5).
Full table
Full table
Conclusions
We demonstrated that hyponatremia was apparent in 16.3% of SCLC, and that hyponatremia was significantly associated with poorer prognosis. To the best of our knowledge, this study with largest number to detect the frequency of hyponatremia and its prognosis value in SCLC.
Hyponatremia in SCLC patients is usually caused by the SIADH (14). In SIADH there is an increased release of ADH which causes excessive water reabsorption in the collecting ducts and a resulting dilutional hyponatremia. There are 4 mechanisms for excessive ADH release: ectopic ADH secretion (release by tumor tissue); increased hypothalamic production of ADH-like substances in neurological disorders (brain tumor); administration of drugs (cytotoxic agents); and finally administration of exogenous ADH or oxytocin. Other factors may cause hypovolemic hyponatremia, including diarrhea or vomiting caused by chemotherapy, radiotherapy and so on (15).
Several retrospective studies have shown the presence of hyponatremia to be a negative prognostic factor. Hansen et al. reported the occurrence rate of hyponatremia in 453 patients with SCLC. It is the first to show that the patients who did not fully normalize the blood sodium value had a worse prognosis than the patient with hyponatremia (8). Hermes et al. conducted a retrospective single institution analysis with 395 cases (9). It reported hyponatremia was present in 18.9% of all patients. Then the occurrence of hyponatremia was 16.3% in our study of 631 patients. They illustrated that hyponatremia was associated with statistical significant shorter median survival than normal group (9.0 vs. 13.0 months, P<0.001) (9). Following our statistical analyses, analysis of the whole population between two groups showed 11.4 vs. 14.5 months of median survival (P<0.001). Our study was consistent with the results of his research.
And in a multivariate Cox regression analysis, the results illustrated that hyponatremia was a significant predictor for survival. Besides sodium levels, LDH, performance, live metastasis and gender were the other variables which also were statistically proven to be significant as predictive factors. According to other literatures (9), gender may be independent prognostic factor and female had a longer survival than man. However, it remains controversial. In our study, female had a shorter survival. It was a retrospective study and the number of female patients was less than male patients. On the other hand, we analyzed that female patients experience more chemotherapy treatment related toxicity and different physical and biological factors could be the reason for the difference between the survivals.
In subnormal analysis of sodium group, the 66 patients who appeared hyponatremia before treatment received sodium supplementation and chemotherapy, 42 cases returned to normal but the values remained subnormal in 24 patients. In the group of 37 patients who appeared hyponatremia during treatment, after receiving sodium supplementation during treatment, 10 patients returned to normal but 27 patients were still hyponatremia. Thereby, normal sodium values could be restored in 49.5% (52/103) in all patients. On the other hand, patients who still remained low sodium values despite adequate treatment were prone to have shorter median survival than the group of patients with complete response (8.70 vs. 14.55 months, P=0.003). These findings are in line with other reports on hyponatremia in SCLC. As Hermes et al. reported, patients with falling sodium values despite adequate treatment were prone to rapid disease progression with median survival below three months (9). Ma et al. also illustrated that the group of patients who was still had low sodium levels in the blood after treatment had worse survival (6.2 vs. 10.3 months) (16).
To our knowledge, our study was the first to report severe hyponatremia had poorer survivals. The group with severe hyponatremia (≤129 mmol/L) showed a more pronounced effect on survival. These patients who had a median survival of only 8.63 months compared to 13.63 months, with the slightly subnormal sodium serum level. The median survival of pre-treatment 66 patients was shorter than the 37 patients during treatment (9.33 vs. 13.40 months, P=0.017). The hyponatremia of patients who appeared pre-treatment hyponatremia was due to the SCLC itself. Whereas, the hyponatremia happened during treatment was mainly caused by chemotherapy or radiotherapy or other factors.
By further analysis in the group of 66 patients with pre-treatment hyponatremia, the median survival of severe hyponatremia was worse (9.03 vs. 12.97 months, P=0.019). Likewise, the group of 37 patients that hyponatremia was present during treatment had similar results (4.40 vs. 13.64 months, P<0.0001). After adjustment for known confounders, the degree of hyponatremia (P=0.007) was a significant predictor for survival in the group of hyponatremia. So, our study implicated that the degree of hyponatremia might be very important in the prognosis for SCLC.
Limitations
As a retrospective analysis, our report had some limitations. The information on treatments for patients with hyponatremia was not very comprehensive. Hence, every patient should have a serum sodium test at every stage of the treatment process. Monitoring the sodium level is required not only before treatment, but also during the oncological treatment.
Conclusions
We demonstrated that hyponatremia was significantly associated with poorer prognosis in SCLC regardless of extensive or limited stage. The value of hyponatremia in SCLC is as a candidate prognosis factor in clinical practice.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board. Written informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0.
References
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78-98. [PubMed]
- Bremnes RM, Sundstrom S, Aasebø U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer 2003;39:303-13. [PubMed]
- Castillo JJ, Vincent M, Justice E. Diagnosis and management of hyponatremia in cancer patients. Oncologist 2012;17:756-65. [PubMed]
- Esposito P, Piotti G, Bianzina S, et al. The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options. Nephron Clin Pract 2011;119:c62-73; discussion c73.
- Platania M, Verzoni E, Vitali M. Hyponatremia in cancer patients. Tumori 2015;101:246-8. [PubMed]
- Cerny T, Blair V, Anderson H, et al. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer 1987;39:146-9. [PubMed]
- Osterlind K, Andersen PK. Prognostic factors in small cell lung cancer: multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res 1986;46:4189-94. [PubMed]
- Hansen O, Sørensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010;68:111-4. [PubMed]
- Hermes A, Waschki B, Reck M. Hyponatremia as prognostic factor in small cell lung cancer--a retrospective single institution analysis. Respir Med 2012;106:900-4. [PubMed]
- Tai P, Yu E, Jones K, et al. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) in patients with limited stage small cell lung cancer. Lung Cancer 2006;53:211-5. [PubMed]
- Tiseo M, Buti S, Boni L, et al. Prognostic role of hyponatremia in 564 small cell lung cancer patients treated with topotecan. Lung Cancer 2014;86:91-5. [PubMed]
- Sagman U, Maki E, Evans WK, et al. Small-cell carcinoma of the lung: derivation of a prognostic staging system. J Clin Oncol 1991;9:1639-49. [PubMed]
- Vincent MD, Ashley SE, Smith IE. Prognostic factors in small cell lung cancer: a simple prognostic index is better than conventional staging. Eur J Cancer Clin Oncol 1987;23:1589-99. [PubMed]
- Hauber HP. Paraneoplastic syndromes in lung cancer. Pneumologie 2011;65:347-58. [PubMed]
- Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer 2007;15:1341-7. [PubMed]
- Ma F, Liu X, Hu C, et al. Clinical features and prognosis analysis of small-cell lung cancer complicated with hyponatremia. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011;36:64-7. [PubMed]


