
Analysis of risk factors associated with breast cancer in women: a systematic review and meta-analysis
Introduction
Breast cancer is one of the common and multiple malignant tumors in women (1). Although China has a low incidence of breast cancer, the incidence of breast cancer has gradually increased, with studies indicating that the incidence among Chinese women will exceed 100 per 100,000 by 2022, and the total number of female breast cancer patients aged 35–49 years will reach 2.5 million by 2022 (2-5). Therefore, studying the risk factors of breast cancer to reduce its incidence is of great significance (6).
The occurrence and development of breast cancer is the result of a combination of factors inside and outside the body. Its occurrence is associated with poor lifestyle factors (e.g., brady, drinking, smoking, hormone, et al.), environmental factors, and social psychological factors. It has been shown that 5–10% of breast cancers can be attributed to factors such as genetic mutations and family history, and 20–30% of breast cancers can be attributed to potentially modifiable factors (e.g., brady, drinking, smoking, hormone, et al.) (7). Breast cancer is the most common cancer in women worldwide and is the leading cause of cancer death among women. In 2018, there were about 2.09 million newly confirmed breast cancer cases in women and about 630,000 deaths. The incidence of breast cancer varies worldwide, but is increasing. Although the incidence of breast cancer (36.1/105) and mortality (8.8/105) is relatively low worldwide, the incidence of breast cancer and mortality in Chinese women ranks first globally due to the large population in China, and has increased in recent years (17.6% and 15.6%, respectively) (8). As the incidence of breast cancer continues to increase worldwide, so does disease burden, which has become a major global public health problem (9).
Breast cancer is a multifactorial disease that mainly includes genetic factors, environmental factors, and behavioral lifestyle factors (10). The aim of the present review was to explore the epidemiology and related risk factors of breast cancer worldwide to understand its prevalence and to help in early detection. The main risk factors for breast cancer are genetic factors, namely family history; diet and obesity, as the quality of life in our country improves, women are getting more and more obese, and their diet tends to be more and more high-fat; smoking and drinking; the other is ionizing radiation; still have namely menstruation, bear and whether lactation, these factors also can affect the occurrence of breast cancer; there is a great relationship between breast cancer and the change of estrogen inside the body. In life, we should avoid using cosmetics containing estrogen as far as possible to reduce the influence of exogenous hormones on the body. There’s been a lot of controversy around these appeals. Therefore, it is necessary to systematically review the risk factors of breast cancer by using meta methods to guide clinical prevention and treatment.
Although Chinese scholars have conducted meta-analyses of breast cancer risk factors, the included research literature is limited; therefore, language bias and issues with timeliness will occur. In the present study, we conducted a meta-analysis of breast cancer risk factors in Chinese women by collecting relevant literature from 2001 to 2021 to provide basic data for the prevention of breast cancer in Chinese women. We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-193/rc).
Methods
Search strategy
We searched both Chinese and international databases, including Chongqing Web, Wanfang, Medline, PubMed, organizations (The International breast cancer Center was established in Bibb, Meager breast cancer Center, et al.), registers of clinical trials (www.medresman.org) and websites (International Association of breast cancer, American Society of breast cancer for Woman, et al.) and Science Direct using the following keywords: “Breast cancer”, “Breast neoplasms”, “Risk factors”, “Breast tumor”, “Prognostic”, et al.
Inclusion criteria
(I) The patients were breast cancer patients diagnosed by pathological cytology, and the control group comprised patients who lived in the same area with the patient and had an age difference of less than 5 years, who were admitted to the hospital at the same time based on a large community population or the same hospital with similar conditions. (II) The articles focused on the relationship between risk factors and breast cancer. (III) All the studies included in this study strictly comply with PICOS principles. PICO is a formatted retrieval method based on evidence-based medicine (EBM) theory. Interventions, outcomes. PICO divided each question into four parts: participants who participated in the interventions, or outcomes.
Exclusion criteria
The exclusion criteria were as follows: (I) duplicate articles; (II) only an abstract but no full text; (III) no control group; (IV) review articles; and (V) studies with inaccurate design methods, low reliability, and poor quality (Figure 1).
Statistical analysis
Statistical analysis was performed using Review Manager 5.1 (Chicago, USA). Using the OR value of each study as the effect index, ln OR and its SE, SE = log (upper 95% CI/95% CI /95% CI)/(1.962) and the OR values of each risk factor in each study by OR and its 95% CI; heterogeneity using Q test and I2 (P<0.1 Show heterogeneity), where I2 is 0% to 40% indicate heterogeneity is unimportant, 30% to 60% indicate possible moderate heterogeneity, 50% to 90% indicate substantial heterogeneity, 75% to 100% indicate considerable heterogeneity, homogeneity and heterogeneity data were analyzed with fixed-effects and random-effects models respectively; sensitivity analysis to compare efficiency by selecting different statistical models. Differences in value point estimates and interval estimates should be combined; publication bias is assessed by the safety factor (NR), which means that at least more “negative” studies are needed to reverse the conclusions of the comprehensive analysis, thus judging the degree of publication bias. In systematic evaluation, any kind of variation between studies is referred to as heterogeneity. Heterogeneity mainly includes three types: (I) clinical heterogeneity: variation of subjects, interventions and outcomes; (II) methodological heterogeneity: diversity and bias risk in study design; (III) statistical heterogeneity: the diversity of intervention effects assessed in different studies is the result of both clinical and methodological heterogeneity.
Publish bias analysis
Publication bias was determined using funnel plots and further quantitative evaluation combined with Begg and Egger tests; all analyses were statistically significant at P<0.05. Funnel plots, the most common method for identifying publication bias in meta-analysis, reflect the estimated intervention effect of a single study with a certain sample size or accuracy. In the absence of bias, points in the image should converge into an inverted funnel. If bias exists, the appearance of funnel plot is not symmetrical and the bottom corner of the graph is blank. In such cases, the effects calculated by meta-analysis may overestimate the efficacy of the intervention. The sensitivity analysis combined the changes of the effect size between the fixed-effects model and the random-effects model to determine whether the analysis results were stable (Figures 2,3).
Results
Literature screening results
A total of 1283 relevant articles were retrieved, including organizations [22], websites [10] and registers [551], and databases [700]. Articles were included/excluded according to the inclusion and exclusion criteria. Duplicate articles were excluded during the initial screening. Twelve articles were finally included after reading the topic, abstract, and full text (11-22). A total of 20,628 breast cancer patients (Table 1). Almost all the literatures included in this study are within the effective range of the triangle, so there is no obvious risk bias.
Table 1
| Study and year | Case (n) | Case/control | Tumor type | Controlled source | Follow-up time (months) | Jadad score |
|---|---|---|---|---|---|---|
| Unlu O, et al. 2017 | 3,325 | 1,621/1,704 | Breast cancer | Hospital | 15.8 | 4 |
| Kabat GC, et al. 2010 | 1,357 | 661/696 | Breast cancer | Crowd | 15.4 | 4 |
| Figueroa JD, et al. 2021 | 514 | 216/488 | Breast cancer | Crowd | 10.4 | 3 |
| Liu YT, et al. 2011 | 1,351 | 669/698 | Breast cancer | Hospital | 18.2 | 5 |
| Hammer J, et al. 2019 | 2,161 | 489/1,672 | Breast cancer | Hospital | 20.1 | 4 |
| Dorjgochoo T, et al. 2008 | 3,452 | 582/2,870 | Breast cancer | Crowd | 12.4 | 5 |
| Newcomer LM, et al. 2003 | 5,510 | 2,342/3,168 | Breast cancer | Hospital | 15.2 | 4 |
| Behravan H, et al. 2020 | 695 | 445/250 | Breast cancer | Crowd | 7.5 | 4 |
| Santen RJ, et al. 2020 | 566 | 238/328 | Breast cancer | Hospital | 11.2 | 4 |
| Hehr T, et al. 2019 | 617 | 322/617 | Breast cancer | Hospital | 6.5 | 5 |
| Hellgren R, et al. 2020 | 214 | 69/145 | Breast cancer | Crowd | 8.5 | 4 |
| Poehls UG, et al. 2019 | 866 | 337/529 | Breast cancer | Hospital | 10.5 | 4 |
History of benign breast disease
A total of 10 studies were included in our study. A systematic review and meta-analysis were conducted on the risk factors of breast cancer. There was no statistically significant difference in benign breast lesions between the experimental group and the blank control group (OR: 1.03, 95% CI: 0.95–1.12, P=0.42) (Figure 4).
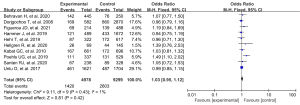
Family history of breast cancer
A total of 8 studies were included in our study. A systematic review and meta-analysis were conducted on the risk factors of breast cancer. There was no statistically significant difference in benign breast lesions between the experimental group and the blank control group (OR: 2.02, 95% CI: 1.83–2.23, P<0.00001) (Figure 5).
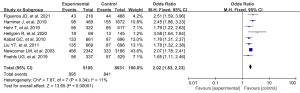
Menopause onset >50 years
A total of 8 studies were included in our study, and a systematic review and meta-analysis were conducted on the risk factors of breast cancer. There was a statistically significant difference between menopausal patients over 50 years old in the experimental group and those in the blank control group (OR: 1.78, 95% CI: 1.62–1.95, P<0.00001) (Figure 6).
Use of oral contraceptives
A total of 6 studies were included in our study. A systematic review and meta-analysis were conducted on the risk factors of breast cancer. There were statistically significant differences between oral contraceptives in the experimental group and blank control group (OR: 1.16, 95% CI: 1.02–1.32, P=0.02) (Figure 7).
Number of at-term pregnancies
A total of 4 studies were included in this study. A systematic review and meta-analysis were conducted on the risk factors of breast cancer. There were statistically significant differences between full-term pregnancy in the experimental group and the blank control group (OR: 0.80, 95% CI: 0.66–0.97, P=0.03) (Figure 8).
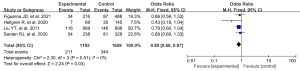
Breastfeeding
A total of 6 studies were included in our study. A systematic review and meta-analysis were conducted on the risk factors of breast cancer. Postpartum breastfeeding in the experimental group was significantly different from that in the blank control group (OR: 0.84, 95% CI: 0.74–0.96, P=0.01) (Figure 9).
Discussion
Meta-analysis was first proposed by Glass in 1976 as a comprehensive analysis using a scientific, systematic approach to a series of independent studies with the same purpose (23). This method can evaluate the inconsistency of the research results and quantitatively evaluate the size of the effect, test the hypothesis, find the shortcomings of previous studies, and re-analyze the literature data. The meta-analysis method can improve the statistical efficiency of the original results, resolve inconsistencies in study results, and improve effect estimates (24).
Our study provides a comprehensive analysis of the history of benign breast gland, family history of breast cancer, menopause onset >50 years, and use oral contraceptives as risk factors for breast cancer. This finding is consistent with previously published studies. Previous studies (25,26) indicate that a family history of breast cancer is an important factor affecting breast cancer development; mutations in the p53 gene and the BReast CAncer (BRCA)-1 and BRCA-2 genes are all related to breast cancer pathogenesis. Our findings indicate that the onset of breast cancer is characterized by familial agglomeration. There is an association between risk of benign breast disease and its histological type. Previous studies (27,28) have confirmed that, after improving the risk assessment, there is a relationship between the onset of breast cancer and specific benign breast diseases, including sclerosing breast disease, breast fibromas, and breast papillomas. The good result of menopausal onset >50 years as a risk factor for breast cancer suggests that breast cancer onset is associated with endogenous estrogen levels. The results of previous studies (29,30) suggested that oral contraceptives increase the risk of premenopausal breast cancer. Although the results of our study cannot directly indicate whether oral contraceptives increase the risk of premenopausal or postmenopausal breast cancer, the comprehensive analysis indicates that oral contraceptives are a risk factor for breast cancer. Breastfeeding and the number of at-term pregnancies were found to be protective factors for breast cancer. Therefore, breastfeeding should be advocated to prevent breast cancer and for maternal and infant health.
As meta-analysis is a statistical synthesis of the results of the original study, it does not only exclude the bias in the original study but also introduces new bias if not properly handled during the literature search and selection. The findings of our study indicated that a healthy history of benign breast disease, family history of breast cancer, menopause onset >50 years, use oral contraceptives and the quantitative index of publication bias of 1 fetus was large. The 12 articles included in our study, matching some possible confounders and reducing the impact of confounders.
The present study has some limitations in the research process. First, the included studies were all retrospective controlled studies with a greater probability of selection bias and could affect the concluding value of the meta-analysis. Most studies did not directly report the hazard ratio and its 95% CI, and the data extracted from the survival curve could be biased from the real data, which might bias the merger results.
We found that menstrual regularity, lactation, and physical exercise could be protective factors for the onset of breast cancer in women, consistent with the results. As a controllable protective factor of breast cancer, breastfeeding and physical exercise should be widely advocated. However, in the present study, we found no association between menarche age of 14 years, menopausal status, and fertility with breast cancer onset in women. Studies have shown that the occurrence of breast cancer is related to the stimulating effect of hormones, and menstrual status and birth history can also lead to altered hormone levels. Previous studies (24-27) have shown that early menarche age, infertility, and menopause increase the risk of breast cancer, whereas others have shown that premenopausal women have a higher risk of breast cancer (27-30). Further analytical studies are therefore needed.
As the onset of breast cancer is a common consequence of multiple risk factors, more rigorous-design, detailed, high-quality epidemiological and biological studies should be conducted in the future to further confirm the association between breast cancer and multiple risk factors.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-193/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-193/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Cicco P, Catani MV, Gasperi V, et al. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019;11:1514. [Crossref] [PubMed]
- Dydjow-Bendek D, Zagożdżon P. Selected dietary factors and breast cancer risk Przegl Epidemiol 2019;73:361-8. [Crossref] [PubMed]
- Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 2019;177:537-48. [Crossref] [PubMed]
- Escala-Garcia M, Morra A, Canisius S, et al. Breast cancer risk factors and their effects on survival: a Mendelian randomisation study. BMC Med 2020;18:327. [Crossref] [PubMed]
- Slepicka PF, Cyrill SL, Dos Santos CO. Pregnancy and Breast Cancer: Pathways to Understand Risk and Prevention. Trends Mol Med 2019;25:866-81. [Crossref] [PubMed]
- Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol 2019;12:140. [Crossref] [PubMed]
- Migliavacca Zucchetti B, Peccatori FA, Codacci-Pisanelli G. Pregnancy and Lactation: Risk or Protective Factors for Breast Cancer? Adv Exp Med Biol 2020;1252:195-7. [Crossref] [PubMed]
- Solikhah S, Promthet S, Hurst C. Awareness Level about Breast Cancer Risk Factors, Barriers, Attitude and Breast Cancer Screening among Indonesian Women Asian Pac J Cancer Prev 2019;20:877-84. [Crossref] [PubMed]
- Arthur RS, Wang T, Xue X, et al. Genetic Factors, Adherence to Healthy Lifestyle Behavior, and Risk of Invasive Breast Cancer Among Women in the UK Biobank. J Natl Cancer Inst 2020;112:893-901. [Crossref] [PubMed]
- Zehr KR. Diagnosis and Treatment of Breast Cancer in Men. Radiol Technol 2019;91:51M-61M. [PubMed]
- Unlu O, Kiyak D, Caka C, et al. Risk factors and histopathological features of breast cancer among women with different menopausal status and age at diagnosis. J BUON 2017;22:184-91. [PubMed]
- Kabat GC, Jones JG, Olson N, et al. Risk factors for breast cancer in women biopsied for benign breast disease: a nested case-control study. Cancer Epidemiol 2010;34:34-9. [Crossref] [PubMed]
- Figueroa JD, Gierach GL, Duggan MA, et al. Risk factors for breast cancer development by tumor characteristics among women with benign breast disease. Breast Cancer Res 2021;23:34. [Crossref] [PubMed]
- Liu YT, Gao CM, Ding JH, et al. Physiological, reproductive factors and breast cancer risk in Jiangsu province of China. Asian Pac J Cancer Prev 2011;12:787-90. [PubMed]
- Hammer J, Geinitz H, Nieder C, et al. Risk Factors for Local Relapse and Inferior Disease-free Survival After Breast-conserving Management of Breast Cancer: Recursive Partitioning Analysis of 2161 Patients. Clin Breast Cancer 2019;19:58-62. [Crossref] [PubMed]
- Dorjgochoo T, Deming SL, Gao YT, et al. History of benign breast disease and risk of breast cancer among women in China: a case-control study. Cancer Causes Control 2008;19:819-28. [Crossref] [PubMed]
- Newcomer LM, Newcomb PA, Trentham-Dietz A, et al. Oral contraceptive use and risk of breast cancer by histologic type. Int J Cancer 2003;106:961-4. [Crossref] [PubMed]
- Behravan H, Hartikainen JM, Tengström M, et al. Predicting breast cancer risk using interacting genetic and demographic factors and machine learning. Sci Rep 2020;10:11044. [Crossref] [PubMed]
- Santen RJ, Heitjan DF, Gompel A, et al. Underlying Breast Cancer Risk and Menopausal Hormone Therapy. J Clin Endocrinol Metab 2020;105:dgaa073.
- Hehr T, Baumann R, Budach W, et al. Radiotherapy after skin-sparing mastectomy with immediate breast reconstruction in intermediate-risk breast cancer: Indication and technical considerations. Strahlenther Onkol 2019;195:949-63. [Crossref] [PubMed]
- Hellgren R, Saracco A, Strand F, et al. The association between breast cancer risk factors and background parenchymal enhancement at dynamic contrast-enhanced breast MRI. Acta Radiol 2020;61:1600-7. [Crossref] [PubMed]
- Poehls UG, Hack CC, Wunderle M, et al. Awareness of breast cancer incidence and risk factors among healthy women in Germany: an update after 10 years. Eur J Cancer Prev 2019;28:515-21. [Crossref] [PubMed]
- Pullella K, Kotsopoulos J. Arsenic Exposure and Breast Cancer Risk: A Re-Evaluation of the Literature. Nutrients 2020;12:3305. [Crossref] [PubMed]
- Yoo TK, Han KD, Kim D, et al. Hormone Replacement Therapy, Breast Cancer Risk Factors, and Breast Cancer Risk: A Nationwide Population-Based Cohort. Cancer Epidemiol Biomarkers Prev 2020;29:1341-7. [Crossref] [PubMed]
- Rey-Vargas L, Sanabria-Salas MC, Fejerman L, et al. Risk Factors for Triple-Negative Breast Cancer among Latina Women. Cancer Epidemiol Biomarkers Prev 2019;28:1771-83. [Crossref] [PubMed]
- Prusty RK, Begum S, Patil A, et al. Knowledge of symptoms and risk factors of breast cancer among women: a community based study in a low socio-economic area of Mumbai, India. BMC Womens Health 2020;20:106. [Crossref] [PubMed]
- Murphy JD, Sandler D, White AJ, et al. Severe acne and risk of breast cancer. Breast Cancer Res Treat 2019;177:487-95. [Crossref] [PubMed]
- Gupta R, Gupta S, Mehrotra R, et al. Risk factors of breast cancer and breast self-examination in early detection: systematic review of awareness among Indian women in community and health care professionals. J Public Health (Oxf) 2020;42:118-31. [PubMed]
- Shi M, O'Brien KM, Weinberg CR. Interactions between a Polygenic Risk Score and Non-genetic Risk Factors in Young-Onset Breast Cancer. Sci Rep 2020;10:3242. [Crossref] [PubMed]
- Tagoe EA, Dwamena-Akoto E, Nsaful J, et al. High atherogenic index of plasma and cardiovascular risk factors among Ghanaian breast cancer patients. Exp Biol Med (Maywood) 2020;245:1648-55. [Crossref] [PubMed]








