
Alfentanil and propofol induced anesthesia for patients with huge endotracheal tumor undergoing fiberoptic bronchoscopic interventional therapy: case report
Introduction
Tracheal tumor is always a big challenge to anesthesiologists. An appropriate anesthesia scheme should be made according to the size and location of the mass, as well as the clinical manifestation of the patients. For patients with extremely huge endotracheal tumor, a symptom of severe respiration distress is always accompanied, which makes it even more difficult to be dealt with. Specifically, the airway obstruction in the middle and lower part of the trachea cannot be relieved by tracheotomy. At the same time, because of the extreme narrowness of the trachea, it is of high risk and extremely hard to perform endotracheal intubation. Therefore, the technique of extracorporeal membrane oxygenation (ECMO) is recommended to ensure the safe performance of anesthesia and surgery for such patients (1,2). However, an ECMO team is not always available in some hospitals. Here we report the anesthesia management of two patients with extremely huge endotracheal tumor. Under our anesthesia strategy, the symptom of airway obstruction was successfully relieved by fiberoptic bronchoscopic interventional therapy. The experience was reviewed and summarized to provide a reference for the proper treatment for such critically ill patients. We present the following cases in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-199/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
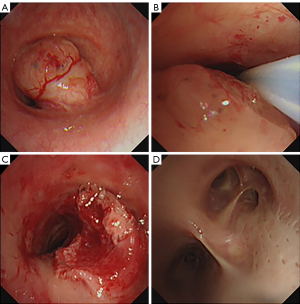
A 65-year-old patient, male, weighing 55 kg, 170 cm in height, complained a symptom of asthma for 2 months. Computed tomography (CT) scan revealed that a mass of 1.7 cm × 1.4 cm protruded into the tracheal cavity from the right posterior wall at the level of the sixth cervical vertebrate. Considering that the tumor located in the middle part of trachea, tracheotomy could not be performed. An emergency fiberoptic bronchoscopic interventional therapy under general anesthesia was adopted. When the patient entered the operating room, he had severe dyspnea and dysphoria with three concave signs. A high flow of oxygen 8 L/min was inhaled with a mask. Vital sign monitoring showed heart rate (HR) 120 bpm, non-invasive blood pressure (NIBP) 110/60 mmHg, SpO2 91% and breath rate (BR) 30 tpm. After nebulized 1% tetracaine solution was inhaled for 10 min, a routine fiberoptic bronchoscopy was performed. Topical anesthesia of the airway was reinforced by spraying 2% lidocaine into the trachea through fiberoptic bronchoscope. It revealed that an extremely huge endotracheal tumor located about 6 cm below the vocal cord, which almost completely blocked the trachea (Figure 1A). The narrowed gap between the tumor and the tracheal wall could be enlarged by pushing the mass away with the fiberoptic bronchoscope (Figure 1B). Concern lied in the high risk of suffocation after the induction of anesthesia, therefore, a spontaneous breath preserved anesthesia scheme without muscle relaxant was adopted. Alfentanil 10 µg/kg and then a continuous infusion at a rate of 5 µg/kg/h was administered. Propofol was given with target-controlled infusion (TCI) mode at an initial plasma concentration of 1 ng/mL, then gradually increased and maintained at a target concentration of 2.0–2.5 ng/mL. Then a size 5 single-tube laryngeal mask (Willy company, Guangzhou, China) was inserted. After the induction, the patient’s HR was 95 bpm and NIBP was 105/55 mmHg. Pure oxygen was inhaled and the tidal volume (VT) of the patient’s spontaneous breath was 250–300 mL, BR was 18 tpm, SpO2 was above 98%. However, when the bispectral index (BIS) value further decreased to 40, severe airway obstruction occurred, and there was no VT manifested on the anesthesia machine. Auxiliary ventilation mode of synchronized intermittent mandatory ventilation (SIMV) failed to ventilate the patient. A fiberoptic bronchoscope was inserted through the laryngeal mask and it showed that the gap between the tumor and the tracheal wall nearly disappeared. The trachea was blocked by the tumor completely. However, by pushing the mass and reopening the gap with the fiberoptic bronchoscope, the patient could breathe spontaneously again. An electrocision maneuver was carried out under fiberoptic bronchoscope subsequently. Pure oxygen was replaced with air at the instant time of electrocision procedure to prevent airway burning. During the 1.5 h of operation, SpO2 was maintained at 95–99%. After the interventional therapy, partial tumor was successfully removed (Figure 1C), and the VT of the patient’s spontaneous breath could reach over 500 mL. The laryngeal mask was extubated safely after the operation. All the vital signs were stable, and the symptom of airway obstruction was relieved (Figure 1D). After regular radiotherapy, the tumor disappeared and the patient achieved a full recovery.
Case 2
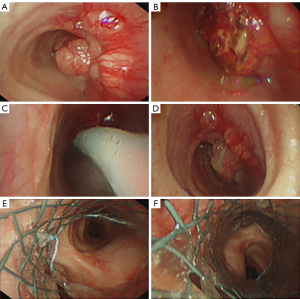
A 42-year-old patient, female, 55 kg and 165 cm in height, complained of hoarseness for half a year and stridor for one week. CT scan showed a mass about 2.5 cm × 3 cm in size located 5 cm below the glottis. A routine fiberoptic bronchoscopy displayed the mass occupied nearly all the space of the trachea. The gap between the tumor and trachea was so narrow that even a biopsy procedure was canceled for the concern of completely airway blockade by bleeding. It was scheduled to remove part of the mass through fiberoptic bronchoscopy preliminarily, then resect the ill segment of the trachea with a surgery. The patient arrived at the operation room with a nasal prong of oxygen flow at 6 L/min. The initial vital signs were NIBP of 120/60 mmHg, HR of 65 bpm and SpO2 of 95%. Nebulized 1% tetracaine solution was inhaled for 10 min to achieve surface anesthesia in upper respiratory tract. Preoxygenation was performed with a high oxygen flow of 10 L/min. When the patient was asked to breathe forcefully, the signs of upper airway obstruction were obvious. Alfentanil 10 µg/kg and propofol 1.5 mg/kg were injected for the induction of general anesthesia. Then a size 4 single-tube laryngeal mask was inserted. After the induction, the anesthesia was maintained with a continuous infusion of propofol 6–8 mg/kg/h and alfentanil 5 µg/kg/h. The patient’s HR was 68 bpm and NIBP was 101/60 mmHg. Pure oxygen was inhaled with a spontaneous and pressure supportive ventilation mode. However, the patient’s VT decreased gradually. Pressure-controlled ventilation (PCV) mode failed to ventilate the patient. A fiberoptic bronchoscope was inserted into the trachea through the laryngeal mask and it revealed that the mass blocked the airway completely (Figure 2A,2B). But by pushing the tumor with fiberoptic bronchoscope, the airway was reopened (Figure 2C). And the patient could breathe spontaneously again with VT over 300 mL. BR was 18 tpm and SpO2 increased to 100%. It was tried to remove the mass with an electroincisor. However, only a very small part of the mass was removed due to the hard textile of the tumor (Figure 2D). Given that there was still a high risk of edema and apnea after the operation, an endotracheal stent was implemented to improve ventilation of the patient (Figure 2E,2F). Then a partial trachea resection surgery was safely carried out and the patient was discharged from the hospital after 2 weeks.
Discussion
The incidence of tracheal tumors in adults is 1 to 2 per 1,000,000 persons and about ninety percent are malignant (3,4). The clinical presentation in the early stage of tracheal tumor is occult (5). Therefore, the diagnosis is usually delayed until the tumor grows huge enough to block the airway severely, resulting in difficult treatment, poor prognosis and high mortality (6,7). Neoplasms can occur at any part from the neck segment of trachea to the proximal carina, but only for tumors in the upper segment of the trachea, tracheotomy can be taken into account (8). Sedatives and muscle relaxants could result in the collapse of the tumor and the neck tissue, leading to complete airway obstruction and even death (9). It is therefore extremely challenging to perform general anesthesia for patients with huge tracheal tumor.
To ensure the safety of patients with dangerous airway obstruction secondary to anterior neck or tracheal disease, the technique of ECMO has been reported to be particularly useful (3,10). However, the implementation of ECMO increases the risk of bleeding and lung dysfunction. A significant neurologic morbidity and mortality are also associated with ECMO therapy (11). And trained medical staffs and special equipment are also needed. Thus the implementation of ECMO assisted tracheal tumor surgery is always difficult to be carried out in some hospitals.
Surgical resection and airway reconstruction are the preferred and definitive therapeutic approaches for tracheal tumor (12). For patients with unresectable tracheal tumor, therapeutic bronchoscopy, cryotherapy, laser ablation and stenting implantation are adjuncts to relieve the symptom of airway obstruction and prolong the period of palliation (13). These techniques should be weighed and selected cautiously depending on their specific merits and shortcomings (14). Therapeutic rigid bronchoscopy (RB) has been an effective tool for restoring airway lumen patency over the past century (15). However, RB-related complications are serious and involve various aspects from rigid bronchoscopic intubation, type of anesthesia implemented, therapeutic intervention performed to characteristics of patients and lesions (16). Therapeutic fiberoptic bronchoscope is another widely used effective technique to offer prompt palliation for patients with endotracheal tumor. A study that included nine patients with severe tracheobronchial stenosis caused by tumor proved that electrosurgery with a fiberoptic bronchoscope and a snare could cause little smoke, take few minutes and have rare complications (17). It has been demonstrated that insertion of a self-expandable endotracheal metal stent through fiberoptic bronchoscope was a comfortable method to provide palliation for inoperable patients with tracheobronchial tumor (18).
General anesthesia is always needed to for the safe performance of fiberopic broncoscopic intervention. It is of great essence to carefully examine the characteristics of the tumor and evaluate the concomitant potential difficulty on ventilation when performing a general anesthesia (19). The size and the location of the mass, the type of tracheal stenosis, the degree of tracheal involvement, the symptoms of patients, and the treatment plan are to be thoroughly reviewed before the induction of general anesthesia.
The two cases described above were extremely huge endotracheal tumors. Both were successfully handled with fiberoptic bronchoscope under general anesthesia. The tumors were located at the middle to lower segment of the trachea. Both patients had severe respiratory distress and relied on the narrow gap between the tumor and the tracheal wall to breathe. Considering the high risk of conventional general anesthesia, a strategy of spontaneous breath preserved general anesthesia induced by propofol and alfentanil was adopted. After the anesthesia induction, both had severe airway obstruction and ventilation failure. However, by pushing the tracheal tumor with fiberoptic bronchoscope, the patient’s airway was reopened and a fairly good oxygenation was achieved by spontaneous breath. Then partial tumor was removed or stent was implemented which guaranteed the following radiologic or surgical therapy.
Since muscle relaxants can lead to loss of upper airway muscle tone and complete obstruction of airway, the use of muscle relaxant was excluded and spontaneous breath was preserved in the two cases. There are a number of advantages in implementing this anesthesia strategy. First, spontaneous breath is a physiological mode which avoids the occurrence of obstruction induced by muscle relaxant administration. And it is also more conducive to the stability of haemodynamics. Second, the spark of electrocision could cause burning injury when pure oxygen is inhaled. Thus a low concentration of oxygen has to be used during the operation. With a narrowed airway tract, oxygen exchange is more effective with a spontaneous breath mode than a mechanical ventilation mode.
Alfentanil and propofol were used for anesthesia induction in the two cases. Alfentanil is a fast effect opioid, and it has the flexibility to change a patient’s level of consciousness due to its short duration of action (20,21). It can provide excellent inhibition of cough reflex to airway stimulation during laryngeal mask insertion nearly without suppression on respiration (22). With the least intense of respiratory and circulatory inhibition, alfentanil is an ideal choice for spontaneous breath preserved general anesthesia (23,24). Propofol is the most commonly used sedative for the induction of general anesthesia with its short acting time, while it can lead to the loss of upper airway muscle tone even in a moderate sedation depth. However, the inhibition on respiration is transient and the loss of upper airway muscle tone can be easily and timely solved by reopening the airway with fiberoptic bronchoscope. From our two cases, it was suggested that 10 µg/kg alfentanil combined with 1.5 mg/kg propofol or a target concentration of 2.0–2.5 ng/mL of propofol with TCI mode could possibly be used for the induction of patients with tracheal tumor. The combination use of alfentanil and propofol could smooth the insertion of laryngeal mask and preserve patient’s spontaneous breath. Respiration inhibition is transient and patient’s spontaneous breath recovers soon after anesthesia induction. There is one point should be noted that inhalation of nebulized 1% tetracaine solution and administration of alfentanil and propofol could only partially inhibit the cough reflex of the trachea. The stimulus of fiberoptic bronchoscope to the trachea could still result in intense cough. Thus topic anesthesia of the trachea should be strengthened by lidocaine spraying through the fiberoptic bronchoscope.
As described above, the incidence of huge endotracheal tumor is rare. And only two cases undergoing fiberoptic bronchoscopic intervention were reported in this paper. The strategy recommended here for the treatment of extremely huge endotracheal tumor should be further confirmed or improved in the future work. It should also be noted that several factors influence the success rate of fiberoptic bronchoscopic treatment of trachea tumor. First, the texture and movement of tumor is related to the possibility of reopening the airway tract when complete blockade of trachea occurs. Thus a routine fiberoptic bronchoscopy should be taken before anesthesia, and the finding that the blockade of the airway could be partly relieved by pushing the tumor with fiberoptic bronchoscope allows the relative safe performance of anesthesia and interventional therapy. Second, the origin of the tumor should be identified properly. Endotracheal tumor is indicated to bronchoscopic interventional therapy. However, tracheal wall compression from outside trachea tumor can’t be relieved by bronchoscopic interventional therapy, but can be solved by endotracheal intubation. Third, the alertness of anesthesiologists and intervention therapists to possible ventilation failure during the operation should be highly emphasized, even for patients without severe symptom of respiratory distress.
Conclusions
For patients with extremely huge endotracheal tumor, an assessment should be made on whether the respiratory function could be improved by fiberoptic bronchoscopic maneuver. And spontaneous breath preserved general anesthesia is strongly suggested. Drugs with the least inhibition on respiratory and circulatory function such as alfentanil and propofol is always helpful. When there is a complete obstruction of the airway by the tumor after the induction of general anesthesia, reopening the respiratory tract by pushing the tumor with fiberoptic bronchoscope may be the most effective and life saving measure for this serious complication.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81770824) and Natural Science Foundation of Hubei Province (No. 2020CFB797).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-199/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-199/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim JJ, Moon SW, Kim YH, et al. Flexible bronchoscopic excision of a tracheal mass under extracorporeal membrane oxygenation. J Thorac Dis 2015;7:E54-7. [PubMed]
- Misra S, Behera BK, Preetam C, et al. Peripheral Cardiopulmonary Bypass in Two Patients With Symptomatic Tracheal Masses: Perioperative Challenges. J Cardiothorac Vasc Anesth 2021;35:1524-33. [Crossref] [PubMed]
- Qiu Y, Chen Q, Wu W, et al. Extracorporeal membrane oxygenation (ECMO)-assisted intratracheal tumor resection and carina reconstruction: A safer and more effective technique for resection and reconstruction. Thorac Cancer 2019;10:1297-302. [Crossref] [PubMed]
- Honings J, Gaissert HA, van der Heijden HF, et al. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 2010;130:763-72. [Crossref] [PubMed]
- Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg 2001;19:339-45. [Crossref] [PubMed]
- Wu CC, Shepard JA. Tracheal and airway neoplasms. Semin Roentgenol 2013;48:354-64. [Crossref] [PubMed]
- Hoerbelt R, Padberg W. Primary tracheal tumors of the neck and mediastinum: resection and reconstruction procedures. Chirurg 2011;82:125-33. [Crossref] [PubMed]
- Li Y, Peng A, Yang X, et al. Clinical manifestation and management of primary malignant tumors of the cervical trachea. Eur Arch Otorhinolaryngol 2014;271:225-35. [Crossref] [PubMed]
- Smeltz AM, Bhatia M, Arora H, et al. Anesthesia for Resection and Reconstruction of the Trachea and Carina. J Cardiothorac Vasc Anesth 2020;34:1902-13. [Crossref] [PubMed]
- Matsuoka Y, Tanaka S, Hirabayashi T, et al. Anesthetic Management with V-V ECMO in a Patient with Severe Tracheal Stenosis. Masui 2016;65:142-5. [PubMed]
- Xie A, Lo P, Yan TD, et al. Neurologic Complications of Extracorporeal Membrane Oxygenation: A Review. J Cardiothorac Vasc Anesth 2017;31:1836-46. [Crossref] [PubMed]
- Wood DE. Bronchoscopic preparation for airway resection. Chest Surg Clin N Am 2001;11:735-48. [PubMed]
- Wood DE. Management of malignant tracheobronchial obstruction. Surg Clin North Am 2002;82:621-42. [Crossref] [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respir Rev 2020;29:190178. [Crossref] [PubMed]
- Chaddha U, Murgu S. Complications of rigid bronchoscopy. Respirology 2021;26:14-8. [Crossref] [PubMed]
- Sagawa M, Sato M, Takahashi H, et al. Electrosurgery with a fiberoptic bronchoscope and a snare for endotracheal/endobronchial tumors. J Thorac Cardiovasc Surg 1998;116:177-9. [Crossref] [PubMed]
- Coolen D, Slabbynck H, Galdermans D, et al. Insertion of a self-expandable endotracheal metal stent using topical anaesthesia and a fibreoptic bronchoscope: a comfortable way to offer palliation. Thorax 1994;49:87-8. [Crossref] [PubMed]
- Wendi C, Zongming J, Zhonghua C. Anesthesia airway management in a patient with upper tracheal tumor. J Clin Anesth 2016;32:134-6. [Crossref] [PubMed]
- Miner JR, Gray R, Delavari P, et al. Alfentanil for procedural sedation in the emergency department. Ann Emerg Med 2011;57:117-21. [Crossref] [PubMed]
- Moman RN, Mowery ML, Kelley B. Alfentanil. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022.
- Ang S, Cheong KF, Ng TI. Alfentanil co-induction for laryngeal mask insertion. Anaesth Intensive Care 1999;27:175-8. [Crossref] [PubMed]
- Reitz JA. Alfentanil in anesthesia and analgesia. Drug Intell Clin Pharm 1986;20:335-41. [Crossref] [PubMed]
- Maguire A, Thompson JP, Guest C, et al. Comparison of the effects of intravenous alfentanil and esmolol on the cardiovascular response to double-lumen endobronchial intubation. Anaesthesia 2001;56:319-25. [Crossref] [PubMed]


