
Significant efficacy of paclitaxel plus carboplatin (TP) as a neoadjuvant regimen for metaplastic squamous cell carcinoma of the breast: a rare case report and literature review
Introduction
Metaplastic squamous cell carcinoma of the breast (MSCCB) is a rare tumor that exists as one of the subgroups of metaplastic breast carcinoma, which accounts for less than 0.1% of all breast carcinomas (1). Since MSCCB is a very invasive tumor, its prognosis is poor (2). Besides, the cause and underlying mechanism of MSCCB remain unclear as a result of few clinical case reports and a lack of standard treatment guidelines.
There are small series of reports of neoadjuvant chemotherapy for MSCCB in the literature. In this case, we reported a MSCCB patient who received neoadjuvant chemotherapy consisting of four cycles of paclitaxel and carboplatin (TP), with a postoperative pathology result reporting a Miller-Payne grade 4. Therefore, we herein report our experience with TP regimens in the case of a MSCCB and discuss the relevant literature. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-484/rc).
Case presentation
A 48-year-old female was first presented with an occasionally found mass in the left breast in 2019. There was no previous family history of cancer. Physical examination found a 3.0 cm × 2.0 cm mass and skin ulcer in the lower quadrant of the left breast, being the ulcer with an uneven surface and bleeding (Figure 1A). The right breast was normal, and there were no palpable lymph nodes in the bilateral axilla.
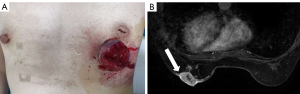
Magnetic resonance imaging (MRI) bilateral breasts showed an irregular mass with long T1 and long T2 in the lower quadrant of the left breast, classified as BI-RADS category 5 lesions. Pricks were seen at the edge of the mass, and part of the skin surface was highlighted. The enhancement scanning was unevenly enhanced (Figure 1B). The serum tumor marker levels were within the normal ranges: Ferroprotein 87.2 µg/L, CA19-9 6.80 U/mL, CA15-3 16.3 U/mL, CA125 18.0 U/mL.
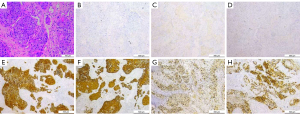
In order to make a definitive diagnosis, we performed a breast biopsy with an ultrasound-guided core needle biopsy, and the diagnosis of metaplastic squamous cell carcinoma was suggested. In addition, immunohistochemical staining revealed that the tumor cells were negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2/neu). However, it was strongly positive for Cytokeratin (CK), Cytokeratin 5/6 (CK5/6), and P53. Besides, the Ki-67 proliferative index was 60% (Figure 2). Further imaging confirmed that there was no evidence of distant metastasis.
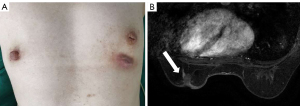
MSCCB was an aggressive disease that behaved like poorly differentiated breast adenocarcinoma, and its clinicopathologic features were similar to the TNBC (3). Guidelines recommend the TP (paclitaxel and carboplatin) regimen as neoadjuvant chemotherapy for TNBC (4). In addition, TP regimen is often used in squamous cell carcinoma of other tissues and organs, such as lung squamous cell carcinoma (5), skin squamous cell carcinoma (6). So we chose TP regimen for this patient. Then, the patient received neoadjuvant chemotherapy based on paclitaxel (175 mg/m2) and carboplatin (AUC 6), and efficacy evaluation every 2 cycles (21 days for 1 cycle). After 4 cycles, the left breast mass was significantly reduced, and the ulcer was healed after 4 cycles of treatment (Figure 3A). MRI examination revealed that the mass in the lower quadrant of the left breast was smaller than before, and the structure was disordered (Figure 3B), indicating a therapeutic effect of partial remission (PR). Then, neoadjuvant chemotherapy was suggested, but the patient refused due to the significantly decreased tumor size, and she requested surgical treatment. Therefore, a modified radical mastectomy with axillary clearance was performed. Postoperative pathology examination showed Miller-Payne grade 4 and no metastasis in the axillary lymph nodes (0/13). These results indicated that neoadjuvant chemotherapy had a good effect. Since then, the patient has been on regular telephone follow-up, and no signs of disease recurrence have been detected within 2 years (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MSCCB is a rare invasive tumor, which often occurs in middle-aged and older women with poor prognoses (7). Zhu et al. (7) analysis of 686 breast squamous cell carcinoma cases revealed a 5-year and 10-year overall survival of 62.1 % and 50.6 %, respectively. MSCCB usually begins with painless breast lumps, typically cystic or solid, and grows rapidly. Some MSCCB patients may have a liquefiable center caused by necrosis, which manifests as skin swelling, ulceration, pain, and other mastitis symptoms (8). Although breast squamous cell carcinoma can invade the skin, lymph node involvement is extremely rare (9). In this case, the patient presented a breast mass that had ulcerated, without lymph node metastasis, when she visited our hospital.
Although the etiology for MSCCB is not clearly defined, most authors believe it is caused by chronic cysts of the breast or squamous metaplasia of the breast ductal epithelium (10). Recent report indicated that breast squamous cell carcinoma might closely correlate with breast implants (11).
MSCCB is easily misdiagnosed or missed due to its rarity and lack of characteristic imaging features (12). The patient’s MRI revealed a high-density mass with irregular and spiculated margins. This is similar to the imaging findings of invasive breast cancer. Consequently, preoperative diagnosis is challenging without biopsy support. Pathological examination of MSCCB showed that more than 90% of the tumor tissues were squamous cell components. In well-differentiated cell carcinomas, some bridges and keratinized cancer pearl were seen. It typically presents as triple-negative and high Ki-67 index (13). CK5/6 positive suggests that the tumor originates from the squamous epithelium. Additionally, the metastasis of other organs should be excluded in the diagnosis of this malignant tumor, and it should also be differentiated from breast invasive ductal carcinoma, breast angiosarcoma, and spindle cell carcinoma.
Up until now, the treatment of MSCCB has not been standardized due to its rarity. The analysis of previous studies found that the most common treatment for MSCCB is surgical excision of the tumor, combined with a comprehensive treatment program of adjuvant chemotherapy. Anne et al. (14) reported that a patient with breast squamous cell carcinoma received only surgical treatment, and no recurrence or metastasis was observed after 36 months of follow-up. If chemotherapy or radiotherapy can be supplemented, the therapeutic effect may be better. There is no uniform standard chemotherapy for patients with MSCCB, but it generally falls into two categories: (I) anthracycline-based chemotherapy (10); and (II) platinum-based chemotherapy (15). Details are shown in Table 1. Alan et al. (25) reported a case of pathologic complete tumor response using TAC (Paclitaxel, epirubicin, and cyclophosphamide) as neoadjuvant chemotherapy. However, some scholars have pointed out that cyclophosphamide, fluorouracil, and anthracyclines are not sensitive to breast squamous cell carcinoma (24). In addition, Pandey et al. (18) also confirmed that postoperative use of adriamycin and cyclophosphamide was ineffective, leading to disease progression and delayed treatments. Moreover, most breast squamous cell carcinoma is triple-negative. If anthracycline-contained regimens are ineffective, can we refer to other TNBC regimens? In the present patient, the tumor was large and ulcerated, and showed extensive inflammatory cell infiltration, which was not suitable for surgery. Therefore, we chose neoadjuvant chemotherapy to find an appropriate chemotherapeutic regimen.
Table 1
| Case | Age, years | Pathological features | Treatment options | Chemotherapy regimens | Follow-up |
|---|---|---|---|---|---|
| Bhatt et al. (16) | 66 | N/A | Adjuvant chemotherapy | (Cisplatin + ifosfamide + mitomycin-C) 4 cycles | Have an excellent disease response |
| Gupta et al. (17) | 63 | ER(−), PR(−), HER2(−) Ki-67 N/A | Adjuvant chemotherapy | (5FU + epirubicin + cisplatin) 6 cycles | Disease free at 1 year follow-up |
| Pandey et al. (18) | Case 1: 39 | Case 1: N/A | Adjuvant chemotherapy | Case 1: Adriamycin + cyclophosphamide + paclitaxel | Case 1: Disease free at 8 months follow-up |
| Case 2: 53 | Case 2: ER(−), PR(−), HER2(−), Ki-67 N/A | Case 2: (Adriamycin + cyclophosphamide) 2 cycles | Case 2: recurrence and metastasis | ||
| Jakubowska et al. (15) | Case 1: 72 | Case 1: ER(−), PR(−), HER2(−), Ki-67(+, 20%) | Adjuvant chemotherapy | Case 1: (Taxotere + Cisplatin) 5 cycles | Case 1: Distant metastases |
| Case 2: 59 | Case 2: ER(−), PR(−), HER2(−), Ki-67 N/A | Case 2: Cisplatin + 5‑fluorouracil | Case 2: Disease free at 6 months follow-up | ||
| Cha et al. (10) | 48 | ER (−), PR(−), HER2(3+), Ki-67(+,95%) | Adjuvant chemotherapy | Doxorubicin + docetaxel + cyclophosphamide | Disease free at 4 months follow-up |
| Murialdo et al. (19) | 54 | ER(−), PR(−), HER2(3+), Ki-67(+,60%) | Adjuvant chemotherapy | Cisplatin + 5-fluorouracil | Disease free at 28 months follow-up |
| Bhosale et al. (9) | 60 | ER(−), PR(−), HER2 N/A, Ki-67 N/A | Adjuvant chemotherapy | (Paclitaxel + carboplatin) 6 cycles | Not reported |
| Tomasicchio et al. (20) | 39 | ER(−), PR(−), HER2(−), Ki-67(+,45%) | Adjuvant chemotherapy | Nab-paclitaxel | Without disease progression |
| Noda et al. (21) | 34 | ER(−), PR(−), HER2(−)Ki-67(+,>90%) | Adjuvant chemotherapy | (Adriamycin + cyclophosphamide) 4 cycles + paclitaxel 12 cycles | Disease free at 30 months follow-up |
| Shrestha et al. (22) | 18 | ER(−), PR(−), HER2(−)Ki-67(+,70%) | Adjuvant chemotherapy | (Paclitaxel + carboplatin) 6 cycles | Disease free at 9 months follow-up |
| Guo et al. (23) | 55 | ER(−), PR(−), HER2(−)Ki-67(+,50%) | Adjuvant chemotherapy | (Docetaxel + cisplatin) 3 cycles | No recurrent or metastatic |
| Tsung et al. (24) | 50 | ER(−), PR(−), HER2(2+, Fish negative), Ki-67 N/A | Neoadjuvant chemotherapy | (Cyclophosphamide + epirubicin + fluorouracil) 4 cycles | Neoadjuvant therapy was ineffective. |
| Alan et al. (25) | 72 | ER(+, ≤1%), PR(−), HER2(−), Ki-67 N/A | Neoadjuvant chemotherapy | Paclitaxel + epirubicin + cyclophosphamide | Pathological complete response |
| Current study | 48 | ER(−), PR(−), HER2(−)Ki-67(+,60%) | Neoadjuvant chemotherapy | (Paclitaxel + carboplatin) 4 cycles | Miller-Payne 4 |
+/−, the immunohistochemical staining of the tumor cells was positive/negative for certain antigen. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2; N/A, not available.
GeparSixto’s study confirmed that TNBC patients showed an increased pCR rate in response to neoadjuvant chemotherapy with platinum-based chemotherapy (26). The PATTERN study revealed that TP (paclitaxel plus carboplatin) was more effective than the standard-dose CEF-T (cyclophosphamide, epirubicin, fluorouracil, and docetaxel) in adjuvant chemotherapy of TNBC (27). Upon reviewing the literature, we found that the TP regimen is effective for breast squamous cell carcinoma, even for advanced patients. Although the patient did not complete the course of neoadjuvant chemotherapy, evaluation of pathologic responses after the neoadjuvant chemotherapy has achieved a good therapeutic effect. In this way, Shrestha et al. (22) reported a patient with large breast squamous cell carcinoma treated with a TP regimen and achieved long-term disease-free survival. Thus, it can be seen that the TP regimen might be effective for MSCCB. However, the neoadjuvant therapy of this patient has not yet achieved a complete pathological response. Given this, we question how to improve the effectiveness of the treatment?
GBG 69-GeparSepto’s study suggests that NAB-paclitaxel is more effective in improving pCR and DFS than solvent-based paclitaxel in neoadjuvant therapy for early breast cancer (28). Besides, Tomasicchio et al. (20) used NAB-paclitaxel as adjuvant chemotherapy for breast squamous cell carcinoma and achieved a good therapeutic response. Recently, immunotherapy has been rising rapidly and is considered an effective therapeutic. Additionally, the KEYNOTE 522’s study confirmed that TP regimen combined with PD-1 inhibitors in neoadjuvant therapy could significantly improve pCR rate in TNBC patients (29). It suggests that a combination of PD-1 inhibitors may further improve the therapeutic effect of breast squamous cell carcinoma.
In conclusion, MSCCB is a rare malignant tumor and has no established treatment method. However, through the analysis of this case and review of the literature, it is indicated that the TP regimen is an effective therapeutic option for treating MSCCB.
Acknowledgments
Funding: This study was funded by National Natural Science Foundation of China (No. 81860715) and Doctor Foundation of Affiliated Hospital of Zunyi Medical University (No. 201712).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-484/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-484/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qi J, Hu Z, Xiao H, et al. SOX10 - A Novel Marker for the Differential Diagnosis of Breast Metaplastic Squamous Cell Carcinoma. Cancer Manag Res 2020;12:4039-44. [Crossref] [PubMed]
- Lei T, Pu T, Wei B, et al. Clinicopathologic characteristics of HER2-positive metaplastic squamous cell carcinoma of the breast. J Clin Pathol 2022;75:18-23. [Crossref] [PubMed]
- Wang X, Zhang L, Luo J, et al. Postoperative radiotherapy improves overall survival in patients with primary squamous cell carcinoma of the breast. Asia Pac J Clin Oncol 2021;17:454-61. [Crossref] [PubMed]
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol 2021;39:1485-505. [Crossref] [PubMed]
- Kambayashi T, Ri M, Yanagihara K, et al. A case of endobronchial squamous cell lung cancer successfully treated with weekly chemotherapy of carboplatin and paclitaxel. Gan To Kagaku Ryoho 2003;30:841-4. [PubMed]
- Cowey CL, Robert NJ, Espirito JL, et al. Clinical outcomes among unresectable, locally advanced, and metastatic cutaneous squamous cell carcinoma patients treated with systemic therapy. Cancer Med 2020;9:7381-7. [Crossref] [PubMed]
- Zhu L, Chen K. Clinicopathological features, treatment patterns, and prognosis of squamous cell carcinoma of the breast: an NCDB analysis. BMC Cancer 2019;19:26. [Crossref] [PubMed]
- Chatha SS, Bano R, Farooq M, et al. Squamous Cell Carcinoma of the Breast. J Coll Physicians Surg Pak 2018;28:776-8. [PubMed]
- Bhosale SJ, Kshirsagar AY, Deshmukh SJ, et al. Squamous cell carcinoma of the breast. Am J Case Rep 2013;14:188-90. [Crossref] [PubMed]
- Cha N, Wang S, Lv M, et al. Breast Metaplastic Squamous Cell Carcinoma Diagnosed with Fine Needle and Core Biopsy: A Case Study. Am J Case Rep 2018;19:203-6. [Crossref] [PubMed]
- Goldberg MT, Llaneras J, Willson TD, et al. Squamous Cell Carcinoma Arising in Breast Implant Capsules. Ann Plast Surg 2021;86:268-72. [Crossref] [PubMed]
- Hardy BM, Cortina CS, Javidiparsijani S, et al. Hypercalcemia in Metaplastic Squamous Cell Carcinoma of the Breast. Am J Case Rep 2019;20:366-9. [Crossref] [PubMed]
- Han M, Zhang H, Dabbs DJ. Best Practice (Efficient) Immunohistologic Panel for Diagnosing Metaplastic Breast Carcinoma. Appl Immunohistochem Mol Morphol 2021;29:265-9. [Crossref] [PubMed]
- Anne N, Sulger E, Pallapothu R. Primary squamous cell carcinoma of the breast: a case report and review of the literature. J Surg Case Rep 2019;2019:rjz182. [Crossref] [PubMed]
- Jakubowska K, Kańczuga-Koda L, Kisielewski W, et al. Squamous cell carcinoma of the breast as a clinical diagnostic challenge. Mol Clin Oncol 2018;8:587-91. [Crossref] [PubMed]
- Bhatt L, Fernando I. Primary squamous cell carcinoma of the breast: achieving long-term control with cisplatin-based chemotherapy. Clin Breast Cancer 2009;9:187-8. [Crossref] [PubMed]
- Gupta N, Vashisht R, Nimbran V, et al. Primary squamous cell carcinoma of the breast: case report and management decisions. J Cancer Res Ther 2012;8:323-5. [Crossref] [PubMed]
- Pandey A, Joshi K, Moussouris H, et al. Case Reports on Metaplastic Squamous Cell Carcinoma of the Breast and Treatment Dilemma. Case Rep Oncol Med 2019;2019:4307281. [Crossref] [PubMed]
- Murialdo R, Boy D, Musizzano Y, et al. Squamous cell carcinoma of the breast: a case report. Cases J 2009;2:7336. [PubMed]
- Tomasicchio G, Rizzi A, Stucci LS, et al. Metaplastic squamous cell breast cancer: A case report and treatment strategy during covid-19 pandemic. Int J Surg Case Rep 2021;79:405-8. [Crossref] [PubMed]
- Noda H, Yamashita M, Murakami A, et al. A Case of a Rapidly Growing Granulocyte Colony-Stimulating Factor-Producing Squamous Cell Carcinoma of the Breast. Case Rep Oncol 2021;14:1175-81. [Crossref] [PubMed]
- Shrestha S, Shakya P, Kharel S, et al. Primary squamous cell carcinoma of the breast in a young female- A rare ailment. Clin Case Rep 2021;9:e04214. [Crossref] [PubMed]
- Guo T, Chen Z, Xu J, et al. Change of Pathological Type to Metaplastic Squamous Cell Carcinoma of the Breast During Disease Recurrence: Case Report and Literature Review. Front Oncol 2020;10:32. [Crossref] [PubMed]
- Tsung SH. Primary pure squamous cell carcinoma of the breast might be sensitive to Cisplatin-based chemotherapy. Case Rep Oncol 2012;5:561-5. [Crossref] [PubMed]
- Alan O, Telli TA, Ercelep O, et al. A case of primary squamous cell carcinoma of the breast with pathologic complete response after neoadjuvant chemotherapy. Curr Probl Cancer 2019;43:308-11. [Crossref] [PubMed]
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747-56. [Crossref] [PubMed]
- Yu KD, Ye FG, He M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1390-6. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J Clin Oncol 2019;37:2226-34. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]



