
Prognostic risk factors for T1 thoracic esophageal cancer: a retrospective cohort study
Introduction
Esophageal cancer is a malignant tumor with a poor prognosis. According to the American Cancer Society, the incidence of esophageal cancer and its mortality rate in 2020, were ranked seventh and sixth among malignant tumors, respectively; it results in approximately 450,000 deaths annually (1,2). In the Netherlands and several other western countries, Chemoradiotherapy followed by surgical resection is the preferred treatment for esophageal cancer (3). With the progress of genomics and molecular research, as well as the emergence of immunotherapy and targeted therapy, our understanding of esophageal cancer has been improved, so that we can find more new methods (4-7). At the same time, with the progress of surgery (standardization of resection scope and lymph node resection, progress of perioperative care, progress of minimally invasive and robot assisted technology), opportunities are provided to improve tumor and surgical results (8-10). Due to its low invasiveness, chemoradiotherapy has become the standard treatment for patients with locally unresectable esophageal squamous cell carcinoma (ESCC) (11). For patients who are unable or unwilling to undergo surgery, chemoradiotherapy is recommended (12).
In the past two decades, the treatment of esophageal cancer has developed from simple surgery to multimodal treatment (surgery combined with chemotherapy or radiotherapy and chemotherapy) (13). This change has brought great benefits to patients. Five-year survival rates for esophageal cancer have doubled in some high-income countries, according to the International Cancer Benchmark Partnership report (14,15). Data from the National Cancer Database (USA) showed that the survival rate of multimodality therapy was double that of definitive chemoradiotherapy in nonrandomized comparison (16).
In an earlier study, patients treated with surgery alone and radiotherapy alone had 5-year survival rates of 4% and 6%, respectively, with a slight improvement in patient survival (17). A phase II study in Japan found that the 5-year disease-free survival rate of patients with esophageal cancer who received postoperative chemotherapy was better than that of the simple surgery group (55% vs. 45%, P=0.037), and the overall survival rate was also better than that of the simple surgery group (61% vs. 52%, P=0.13) (18). In a similar study in France, the disease-free survival rate in the combined chemotherapy group after surgery was 34%, while that in the simple surgery group was 19% (P=0.003), while the 5-year survival rates were 38% and 24%, respectively (P=0.02) (19). In a German experiment published in 2005 (n=189), the 2-year progression-free survival rate of patients undergoing surgery was higher than that of patients undergoing continuous chemoradiotherapy (64% vs. 40%, P=0.03) (20).
However, for each patient with esophageal cancer, the effectiveness of treatment is affected by stage, tumor location, treatment, and patient’s situation. Therefore, in this study, the independent risk factors for survival and prognosis of patients with T1 thoracic esophageal cancer were studied through the statistics of the data in the Surveillance, Epidemiology, and End Results (SEER) database, and the effect of multi-modal treatment of esophageal cancer was analyzed, to provide more appropriate treatment and improve the survival for patients with T1 thoracic esophageal cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-308/rc).
Methods
Study design
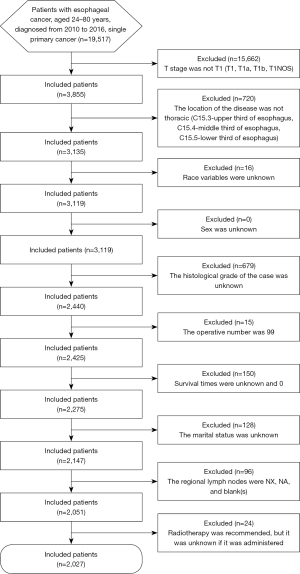
This population-based retrospective cohort study analyzed the data obtained from the SEER database, of 2,027 patients diagnosed with T1 esophageal cancer from 2010 to 2016. The flow diagram of this study is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Sources
The SEER database was established in 1973 and funded by the National Cancer Institute. It is used to record the patients’ demographics (race, sex, age, and marital status), tumor characteristics (pathological type, differentiation degree, and histological type), tumor pathology, treatment (i.e., surgery, radiotherapy, and chemotherapy), prognosis (overall survival rate, disease-specific mortality rate, and cause of death), and other information. The version used in this study was SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying).
Inclusion and exclusion criteria
The data of the following patients were obtained from the SEER database: (I) patients aged 20–84 years; (II) patients who were diagnosed between 2010 and 2016; and (III) patients with esophageal cancer, specifically a single primary cancer.
The exclusion criteria for this study were as follows: (I) race variables were unknown; (II) sex was unknown; (III) the histological grade of the case was unknown; (IV) the location of the disease was not thoracic (thoracic location: C15.3-upper third of the esophagus, C15.4-middle third of the esophagus, C15.5-lower third of the esophagus); (V) the operative number was 99 (indicating that it was unknown if surgery was performed; death certificate only); (VI) survival times were unknown and 0; (VII) the marital status was unknown; (VIII) the American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) stage was not T1 (T1, T1a, T1b, T1NOS); (IX) the regional lymph nodes were NX, NA, and blank(s); (X) the distant metastatic status was NA and blank(s); and (XI) radiotherapy was recommended, but it was unknown if it was administered. A detailed screening flowchart is presented in Figure 2.
Classification and selection of risk factors
According to the SEER database records, the race was classified into white, black, and others (American Indian/AK Native, Asian/Pacific Islander); sex was categorized into male and female; and the marital status was classified into married (including common law) and single (never married), divorced, widowed, separated, unmarried, or having a domestic partner. In terms of tumor classification, this study used the 7th edition of the AJCC TNM staging system: the N stage was categorized into N0, N1, N2, and N3; M stage was classified into M0 and M1; and the histopathological grade was 4, which was further categorized into well-differentiated (Grade I), moderately differentiated (Grade II), poorly differentiated (Grade III), and undifferentiated (undifferentiated; anaplastic; Grade IV). The primary site was classified into upper one-third (C15.3-upper third of the esophagus), middle one-third (C15.4-middle third of the esophagus), and lower one-third (C15.5-lower third of the esophagus) of the esophagus. The pathological types were classified into squamous cell carcinoma (morphology codes: 8000-8046, 8051-8131, 8148-8157, 8230-8249, 8508, 8510-8513, 8560-8570, 8575, 8950, 8980-8981), adenocarcinoma (morphology codes: 8050, 8140-8147, 8160-8162, 8170-8175, 8180-8221, 8250-8507, 8514-8551, 8571-8574, 8576, 8940-8941), and others (morphology code: 8936). Patients were divided into the surgical and non-surgical groups according to whether they had undergone surgery or not.
Statistical methods
Statistical analysis was performed using the R language (R-3.6.1). The propensity score matching (PSM) of nearest neighbor matching method was used to reduce the selection bias between groups. The classification data are expressed as medians and percentiles. A Chi-square test and t-test were used to compare the categorical (ordinal) and continuous variables of patients, tumors, and treatment regimens between the non-surgical and surgical groups, and the data were reported as counts (percentages) and mean ± standard deviation, respectively.
The survival curves were drawn using the Kaplan-Meier method and tested using the log-rank test. Cox’s regression model was used to analyze the independent risk factors for the prognosis of patients with T1 thoracic esophageal cancer. P<0.05 was considered to indicate statistical significance.
Results
Epidemiological characteristics of the patients
A total of 2,027 cases were included in this study. The average age of patients within the surgical and non-surgical groups was 65 (range, 58–71) and 66 years (range, 58–73 years), respectively. The races of the eligible patients were white (86.09%), black (9.08%), and others (4.83%). The proportions of men and women were 17.27% and 82.73%, respectively. The proportions of married and single people (including divorced, among others) were 60.63% and 39.37%, respectively.
The primary site was the lower one-third of the esophagus, accounting for 77.11% of the total cases. The main pathological types were adenocarcinoma and squamous cell carcinoma, accounting for 27.78% and 72.13% of all cases, respectively. Regarding the histological grade, the proportions of well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated carcinomas were 10.16%, 44.45%, 44.01%, and 1.38%, respectively. Table 1 shows the details of the characteristics of patients with esophageal cancer.
Table 1
| Characteristic | Case, n (%) | Non-surgical group 0 (n=1,203) | Surgical group 1 (n=824) | P value |
|---|---|---|---|---|
| Race, n (%) | <0.001 | |||
| White | 1,745 (86.09) | 996 (82.79) | 749 (90.9) | |
| Black | 184 (9.08) | 144 (11.97) | 40 (4.85) | |
| Other | 98 (4.83) | 63 (5.24) | 35 (4.25) | |
| Sex, n (%) | 0.006 | |||
| Female | 1,677 (82.73) | 972 (80.8) | 705 (85.56) | |
| Male | 350 (17.27) | 231 (19.2) | 119 (14.44) | |
| Age (years), median (IQR) | 65 (58, 72) | 66 (58, 73) | 65 (58, 71) | 0.056 |
| YD, n (%) | 0.624 | |||
| 2010 | 352 (17.37) | 211 (17.54) | 141 (17.11) | |
| 2011 | 313 (15.44) | 197 (16.38) | 116 (14.08) | |
| 2012 | 310 (15.29) | 184 (15.3) | 126 (15.29) | |
| 2013 | 361 (17.81) | 215 (17.87) | 146 (17.72) | |
| 2014 | 337 (16.63) | 198 (16.46) | 139 (16.87) | |
| 2015 | 354 (17.46) | 198 (16.46) | 156 (18.93) | |
| Grade, n (%) | <0.001 | |||
| 1 | 206 (10.16) | 55 (4.57) | 151 (18.33) | |
| 2 | 901 (44.45) | 483 (40.15) | 418 (50.73) | |
| 3 | 892 (44.01) | 649 (53.95) | 243 (29.49) | |
| 4 | 28 (1.38) | 16 (1.33) | 12 (1.46) | |
| Histologic, n (%) | <0.001 | |||
| 1 | 563 (27.78) | 441 (36.66) | 122 (14.81) | |
| 2 | 1,462 (72.13) | 762 (63.34) | 700 (84.95) | |
| 3 | 2 (0.1) | 0 (0.00) | 2 (0.24) | |
| Site, n (%) | <0.001 | |||
| 1 | 99 (4.88) | 84 (6.98) | 15 (1.82) | |
| 2 | 365 (18.01) | 253 (21.03) | 112 (13.59) | |
| 3 | 1,563 (77.11) | 866 (71.99) | 697 (84.59) | |
| Marital, n (%) | <0.001 | |||
| Married | 1,229 (60.63) | 662 (55.03) | 567 (68.81) | |
| Single | 798 (39.37) | 541 (44.97) | 257 (31.19) | |
| N 7th, n (%) | <0.001 | |||
| 0 | 1,260 (62.16) | 587 (48.79) | 673 (81.67) | |
| 1 | 661 (32.61) | 532 (44.22) | 129 (15.66) | |
| 2 | 78 (3.85) | 58 (4.82) | 20 (2.43) | |
| 3 | 28 (1.38) | 26 (2.16) | 2 (0.24) | |
| M 7th, n (%) | <0.001 | |||
| 0 | 1,408 (69.46) | 604 (50.21) | 804 (97.57) | |
| 1 | 619 (30.54) | 599 (49.79) | 20 (2.43) | |
| Radiation, n (%) | <0.001 | |||
| Yes | 1,148 (56.64) | 554 (46.05) | 594 (72.09) | |
| No | 879 (43.36) | 649 (53.95) | 230 (27.91) | |
| Chemotherapy, n (%) | <0.001 | |||
| Yes | 930 (45.88) | 372 (30.92) | 558 (67.72) | |
| No | 1,097 (54.12) | 831 (69.08) | 266 (32.28) |
Categorical and continuous variables reported as counts (percentages) and mean ± standard deviation, respectively. IQR, interquartile range; YD, year of diagnosis.
Kaplan-Meier survival analysis
The 3- and 5-year survival rates of the non-surgical group were 66.75% and 33.14%, respectively, whereas those of the surgical group were 61.94% and 27.9%, respectively.
The median survival time was 44 months (range, 42–47 months) and 46 months (range, 43–56 months) in the surgical group and non-surgical group (P=0.27), respectively. For patients with T1 thoracic esophageal cancer, there was no significant difference in the values between the surgical and non-surgical groups. The survival curve is shown in Figure 3.
Multivariate analysis of prognosis
According to Cox’s regression analysis, the results showed that radiotherapy (hazard ratio, 1.46; 95% confidence interval, 1.10–1.93; P=0.008) was an independent risk factor affecting the prognosis, as shown in Table 2.
Table 2
| Factor | Regression coefficient | HR confidence interval | P value |
|---|---|---|---|
| Male | 1.13 | 0.91, 1.39 | 0.267 |
| Moderately differentiated carcinoma | 0.90 | 0.74, 1.09 | 0.290 |
| Poorly differentiated carcinoma | 0.85 | 0.68, 1.06 | 0.143 |
| Undifferentiated carcinoma | 1.16 | 0.56, 2.38 | 0.687 |
| Adenocarcinoma | 0.97 | 0.77, 1.22 | 0.780 |
| Other cancers | 2.57 | 0.34, 19.28 | 0.359 |
| Middle one-third | 1.13 | 0.76, 1.69 | 0.549 |
| Lower one-third | 1.11 | 0.75, 1.64 | 0.595 |
| Single | 0.99 | 0.85, 1.17 | 0.941 |
| N1 | 0.94 | 0.75, 1.18 | 0.606 |
| N2 | 0.83 | 0.51, 1.36 | 0.456 |
| N3 | 0.00 | 0.00, Inf | 0.985 |
| M1 | 1.28 | 0.88, 1.86 | 0.193 |
| Operation | 1.15 | 0.92, 1.44 | 0.230 |
| Radiotherapy | 1.46 | 1.10, 1.93 | 0.008 |
| Chemotherapy | 0.77 | 0.58, 1.03 | 0.073 |
HR, hazard ratio.
The median survival time was 68 months (range, 57–81 months) and 44 months (range, 41–49 months) in the non-radiotherapy and radiotherapy groups, respectively, in the surgery-selected and chemotherapy-treated subgroup. The log-rank test results showed that the P value was 0.00059. For patients with T1 thoracic esophageal cancer, there was a significant difference in the values between the radiotherapy and non-radiotherapy groups. The survival curve is shown in Figure 4.
Discussion
Surgery is the main treatment for patients with stage T1 esophageal cancer. The disadvantage of traditional surgery is that the postoperative complication rate is as high as 30% to 50%. Even in larger hospitals, the mortality rate is still 2% to 3%. Through our research, we found that the median survival of the surgical group (44 months) was slightly lower than that of the non-surgical group (46 months). After the log-rank test, the P value was 0.28 (P>0.05) and was not significant. Therefore, there is no difference between surgery alone and no surgery for patients with T1 thoracic esophageal cancer. Whether postoperative complications are the cause of poor surgical results needs to be studied in the future.
According to COX regression analysis, this study found that race, sex, age, location of onset, or histological type were not independent risk factors for the prognosis of patients with T1 thoracic esophageal cancer. Only radiotherapy was an independent risk factor for the prognosis of patients with T1 thoracic esophageal cancer.
Esophageal cancer is a malignant tumor with a poor prognosis. Multimodal treatments have shown their advantages, but which chemotherapy/radiotherapy regimen is superior is still the subject of large clinical trials. In the subgroup analysis of this study, the median survival time of the non-radiotherapy group was 68 months (range, 57–81 months). The median survival time in the radiotherapy group was 44 months (range, 41–49 months). The log-rank test showed P<0.05. For patients with stage T1 thoracic esophageal cancer, the radiotherapy and non-radiotherapy groups had a significant difference, and the survival of patients was improved. For T1 esophageal cancer patients with surgery and chemotherapy, the absence of radiotherapy is beneficial to improve survival.
The limitations of this study still need to be addressed. First, the SEER database itself has certain limitations as it only includes demographic information, tumor information, treatment information, prognostic information, and other basic factors that have no influence on esophageal cancer but does not include factors such as smoking, drinking, family history of tumors, or dietary environment, thus causing a bias. Secondly, although the SEER database records information on radiotherapy and chemotherapy, this might cause a bias because the order of radio chemotherapy and surgery treatment was not included in the study. Finally, as this study is a retrospective cohort study, the conclusions cannot be sufficiently validated, and further validation through a prospective cohort study is needed for clinical application.
Conclusions
Our data will be useful for clinicians to formulate a possible basis for the clinical treatment of patients with T1 thoracic esophageal cancer to improve their survival and prognosis.
Acknowledgments
We would like to thank Editage (www.editage.cn) for their English language editing.
Funding: This work was supported by the Natural Science Foundation of Anhui Province (No. 1908085QF286); the Natural Science Foundation of Anhui Provincial Department of Education (Nos. KJ2020A0031, KJ2020A0037, KJ2020B15 and KJ2020B12); Open project of Key Laboratory of intelligent optimization and information processing (Minnan Normal University) (No. ZNYH202003); the National Natural Science Foundation of China (No. 61976101).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-308/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-308/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-308/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020;159:335-349.e15. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol 2016;13:348-60. [Crossref] [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Nomura M, Kato K, Ando N, et al. Comparison between neoadjuvant chemotherapy followed by surgery and definitive chemoradiotherapy for overall survival in patients with clinical Stage II/III esophageal squamous cell carcinoma (JCOG1406-A). Jpn J Clin Oncol 2017;47:480-6. [Crossref] [PubMed]
- Kato K, Ito Y, Nozaki I, et al. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients With Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2021;161:1878-1886.e2. [Crossref] [PubMed]
- Hoefnagel SJM, Boonstra JJ, Russchen MJAM, et al. Towards Personalized Treatment Strategies for Esophageal Adenocarcinoma; A Review on the Molecular Characterization of Esophageal Adenocarcinoma and Current Research Efforts on Individualized Curative Treatment Regimens. Cancers (Basel) 2021;13:4881. [Crossref] [PubMed]
- Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019;20:1493-505. [Crossref] [PubMed]
- Morgan E, Soerjomataram I, Gavin AT, et al. International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut 2021;70:234-42. [PubMed]
- Bolger JC, Donohoe CL, Lowery M, et al. Advances in the curative management of oesophageal cancer. Br J Cancer 2022;126:706-17. [Crossref] [PubMed]
- Earlam R, Cunha-Melo JR. Oesophogeal squamous cell carcinoms: II. A critical view of radiotherapy. Br J Surg 1980;67:457-61. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]





