
Development and validation of a prognostic predictive model of pulmonary spindle cell carcinoma from the surveillance, epidemiology and end results database
Introduction
Pulmonary spindle cell carcinoma (PSCC) is a rare type of non-small cell lung cancer (NSCLC). According to the World Health Organization (WHO) classification of lung cancer, PSCC is a subtype of pulmonary sarcomatoid carcinoma (PSC). PSC usually has a poor prognosis and includes five subtypes: pleomorphic carcinoma, pulmonary blastoma, giant cell carcinoma, carcinosarcoma, and spindle cell carcinoma (1,2). Generally, PSC has similar imaging features to other lung cancers (3,4). Hence, the diagnose of PSC relies on immunohistochemistry even molecular abnormality testing from resected specimen, and only a small number of cytological or biopsy specimens can fulfill the need of diagnosis (1,5,6).
PSCC consists of an almost pure population of epithelial spindle cells, with no differentiated carcinomatous elements, and the prevalence of PSCC is low, taking a count of about <1% in all kinds of lung cancer (1,2). The overall survival (OS) of PSC is very short [median OS: 9 months, 95% confidence interval (CI): 7–12 months], and the median survival time of PSCC has not been reported. According to case reports now available, PSCC is a highly malignant tumor with poor prognosis, even in early stage (3,7,8). Qi et al pointed out that most PSCC patients survived less than 24 months after diagnosed; Similarly, after summarizing previous reports, Kida et al mentioned gross PSCC patients had died or relapsed within 1 year, even they therapeutic (3,7-9). So, a study of enough samples, uncovering the prognostic factors of PSCC, is lacked but needed. And until now, there is not a predictive model reliable yet.
As existing studies have come to an agreement on the poor prognosis of PSCC, the choices of its cure are still unclear. Treatment of PSC is usually chosen from surgery, chemotherapy and radiotherapy, while some targeted drugs, programmed death-1 (PD-1) and programmed death ligand 1 (PD-L1) were potential therapies (5,10-14). And previous studies tended to use surgery or chemotherapy treating PSC but still with much dispute (4,15). Parallelly, without enough studies on the PSCC, the therapy of it was decided often referring to PSC or a common lung cancer though its high malignance (16).
Due to the rarity of PSCC, there were few studies except some case reports, small samples’ analysis or exploration on PSC (8,9,16-21). Retrospective or prospective studies with enough samples are necessary, applying survival analysis to reveal the prognostic factors and to provide treatment references of PSCC. And as we talked, there is still much disagreement on if PSCC should be considered and treated as PSC (4,16). Therefore, to establish a retrospective cohort study on PSCC of huge samples, we searched the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (NCI) to conduct a survival analysis, aiming to develop and validate a prognostic model and explore the effective factors related to survival of PSCC. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-427/rc).
Methods
Data source and population
We browsed the NCI’s SEER, using SEER*Stat (8.3.9.2). The SEER program registries routinely collect demographic and clinic data on patients, and the mortality data reported by SEER are provided by the National Center for Health Statistics. Using the 18 Registries database (2000–2018) which covers approximately 27.8% of the U.S. population, we set search option “site and morphology site recode ICD-O-3/WHO2008” as “lung and bronchus” and “site and morphology ICD-O-3 Hist/behav” as “8032/3: spindle cell carcinoma” to established a retrospective cohort. There are 833 patients diagnosed with PSCC in total. According to the diagnosed year, patients are allocated to the primary cohort (diagnosed in 2000–2015) and the validation cohort (diagnosed in 2016–2018). To compare the OS possibilities of PSC, we searched the same database changing “site and morphology ICD-O-3 Hist/behav” as “8032/3: spindle cell carcinoma”, “8022/3: Pleomorphic carcinoma”, “8031/3: Giant cell carcinoma”, “8972/3: Pulmonary blastoma” or “8980/3: Carcinosarcoma, NOS”. All 2,764 patients with PSC were included (Figure 1).
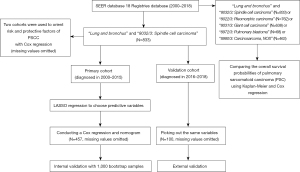
Cut-off date of patients above was December 31 2018, according to the SEER’s manual (submitted in November 2020).
Potential predictive variables
Age, sex, race, site, grade, stage, radiotherapy, surgery, chemotherapy, lymphatic metastasis, size, distant metastasis, and history of malignancy were the potential predictive variables. Age and tumor size were treated as categorical variables when presented in the baseline data (Table 1) but in subsequent statistical analysis, they were regarded as continuous variables.
Table 1
| Variables | Primary cohort [2000–2015] (N=693), N (%) | Validation cohort [2016–2018] (N=140), N (%) |
|---|---|---|
| Age, years | ||
| 20–24 | 3 (0.43) | 1 (0.71) |
| 25–29 | 1 (0.14) | 1 (0.71) |
| 35-39 | 4 (0.58) | 0 (0) |
| 40–44 | 5 (0.72) | 0 (0) |
| 45–49 | 16 (2.31) | 1 (0.71) |
| 50–54 | 21 (3.03) | 5 (3.57) |
| 55–59 | 42 (6.06) | 12 (8.57) |
| 60–64 | 82 (11.83) | 14 (10.00) |
| 65–69 | 81 (11.69) | 18 (12.86) |
| 70–74 | 126 (18.18) | 24 (17.14) |
| 75–79 | 123 (17.75) | 19 (13.57) |
| 80–84 | 113 (16.31) | 22 (15.71) |
| 85+ | 76 (10.97) | 23 (16.43) |
| Sex | ||
| Female | 302 (43.58) | 52 (37.14) |
| Male | 391 (56.42) | 88 (62.86) |
| Race | ||
| White | 603 (87.01) | 112 (80.00) |
| Black | 53 (7.65) | 12 (8.57) |
| Other | 34 (4.91) | 15 (10.71) |
| Unknown | 3 (0.43) | 1 (0.71) |
| Marital status | ||
| Single (never married) | 71 (10.25) | 20 (14.29) |
| Married (including common law) | 356 (51.37) | 74 (52.86) |
| Separated | 7 (1.01) | 0 (0) |
| Divorced | 77 (11.11) | 11 (7.86) |
| Widowed | 142 (20.49) | 31 (22.14) |
| Unknown | 40 (5.77) | 4 (2.86) |
| Site | ||
| Upper lobe | 308 (44.44) | 57 (40.71) |
| Middle lobe | 42 (6.06) | 10 (7.14) |
| Lower lobe | 197 (28.43) | 41 (29.29) |
| Main bronchus | 20 (2.89) | 3 (2.14) |
| Overlapping lesion of lung | 10 (1.44) | 2 (1.43) |
| Unknown | 116 (16.74) | 27 (19.29) |
| Size, mm | ||
| ≤10 | 15 (2.16) | 4 (2.86) |
| ≤20 | 52 (7.50) | 11 (7.86) |
| ≤30 | 56 (8.08) | 9 (6.43) |
| ≤40 | 56 (8.08) | 17 (12.14) |
| ≤50 | 74 (10.68) | 12 (8.57) |
| ≤60 | 61 (8.80) | 11 (7.86) |
| ≤70 | 49 (7.07) | 11 (7.86) |
| >70 | 150 (21.65) | 34 (24.29) |
| Unknown | 180 (25.97) | 31 (22.14) |
| History of malignancy | ||
| No | 512 (73.88) | 95 (67.86) |
| Yes | 181 (26.12) | 45 (32.14) |
| N | ||
| N0 | 314 (45.31) | 73 (52.14) |
| N1 | 48 (6.93) | 8 (5.71) |
| N2 | 185 (26.70) | 32 (22.86) |
| N3 | 38 (5.48) | 9 (6.43) |
| Unknown | 108 (15.58) | 18 (12.86) |
| M | ||
| M0 | 328 (47.33) | 55 (39.29) |
| M1 | 339 (48.92) | 83 (59.29) |
| Unknown | 26 (3.75) | 2 (1.43) |
| Grade | ||
| Well differentiated; grade I | 9 (1.30) | 3 (2.14) |
| Moderately differentiated; grade II | 5 (0.72) | 0 (0) |
| Poorly differentiated; grade III | 252 (36.36) | 27 (19.29) |
| Undifferentiated; anaplastic; grade IV | 61 (8.80) | 7 (5.00) |
| Unknown | 366 (52.81) | 103 (73.57) |
| Stage | ||
| Localized | 119 (17.17) | 26 (18.57) |
| Regional | 154 (22.22) | 23 (16.43) |
| Distant | 394 (56.85) | 86 (61.43) |
| Unknown | 26 (3.75) | 5 (3.57) |
| Radiotherapy | ||
| No | 471 (67.97) | 102 (72.86) |
| Yes | 222 (32.03) | 38 (27.14) |
| Surgery | ||
| No | 493 (71.14) | 116 (82.86) |
| Yes | 193 (27.85) | 23 (16.43) |
| Unknown | 7 (1.01) | 1 (0.71) |
| Chemotherapy | ||
| No | 476 (68.69) | 101 (72.14) |
| Yes | 217 (31.31) | 39 (27.86) |
| Survival time | ||
| Median [IQR] | 4 [1, 16] | 3 [1, 10] |
| Range | 1–218 | 1–32 |
Follow-up cut-off date above was December 31 2018. IQR, interquartile range.
Some variables mentioned above were not directly recorded in an univariable of SEER, so we have combined and adjusted them referring to the eighth edition American Joint Committee on Cancer (AJCC) stage. And considering the variance of 6th, 7th or 8th AJCC stage and lack of available data to adjust the TNM stage as accordant one, we chose the size of the tumor and stage by SEER (localized/regional/distant, Table S1) to act as potential predictive variables instead of T or stage by AJCC. The N and M by AJCC was reserved to describe a tumor’s lymph node and distant metastasis, which were regarded as potential predictive variables.
RX Summ—Surg/Rad Seq and Radiation Recode in SEER were used as references for radiotherapy. If either of the two variables recorded radiotherapy information or radiotherapy mode, it was defined as having received radiotherapy. Otherwise, it was considered as not receiving radiotherapy. Reason no cancer-directed surgery in SEER was used to judge if a patient acquired surgery. Tumor size referred to these SEER variables: CS tumor size (2004–2015), tumor size Summary (2016+), and EOD 10-size (1988–2003). If a record contained approximately data as reminding less than 3 cm (such as in CS tumor Size 2004–2015 variable of SEER), the tumor size was still treated as 3 cm and other analogous size records were treated in the same way. Regional lymph nodes metastasis (N) referred to Derived EOD 2018 N (2018+), Derived AJCC N 6TH Ed (2004–2015), EOD 10-Nodes (1988–2003) and Derived SEER Combined N (2016–2017). Distant metastasis (M) was judged referring to Derived EOD 2018 M (2018+), Derived AJCC M 7th Ed (2010–2015), Derived SEER Combined M (2016–2017), Derived AJCC M 6th Ed (2004–2015) and EOD 10-extent (1988–2003) variables. Stage was derived from the Summary stage 2000 (1998–2017) and Combined Summary Stage (2004+) variables, which was divided into three categories: localized, regional and distant by SEER (the definition of the three categories shown in Table S1).
In the data, we noticed an extreme value of tumor size 88 cm (a 69-year-old male diagnosed in 2014), which may have been misremembered, so its diameter was regarded as a missing value. Survival time less than a month was still counted as 1 month.
The adjusted variables and patients’ demographics and clinical characteristics are shown in Table 1.
Variable selection
We used the Least Absolute Shrinkage and Selection Operator (LASSO) regression to minimize the potential collinearity of variables and select the final predictive variables. The crucial point of LASSO regression is to designate the appropriate λ value. Using data from primary cohort, we made 5-fold cross validation to determine λ value (the minimum one Figure S1). Based on the λ value, we established a Cox regression and chose variables with non-zero coefficients as the predictive variables. In this way, age, sex, stage, surgery, chemotherapy, N, size and history of malignancy were finally chosen to be predictive variables. All above data were only used from the primary cohort (diagnosed in 2000–2015).
Study design and statistical analysis
In this retrospective cohort study, Kaplan-Meier curve was used to compare the survival probabilities, with log-rank test calculating P value. And Cox proportional hazard regression was applied to count the hazard ratio (HR). Chosen by LASSO regression, age, sex, stage, surgery, chemotherapy, N, size and history of malignancy were the predictive variables. These above variables were taken out from the primary cohort and the validation cohort respectively, with missing values omitted. Finally, 457 samples were collected from the primary cohort and 100 samples from the validation cohort (Figure 1). We used the data from the primary cohort to perform a Cox proportional hazard regression and establish a nomogram to predict the OS probability of a PSCC patient in 1-, 3- and 5-year. To measure the nomogram’s performance, we implemented internal and external validation. For internal validation, we used 1,000 bootstrap samples to estimate the concordance index (C-index) and plotted calibration curves to evaluate the deviation between predicted probability and actual probability. The C-index close to 1.0 indicates a perfect prediction, with bigger than 0.7 or 0.8 thought to be a potentially good one, while a 0.5 or smaller one tends to randomly guess. To avoid overfitting, the validation cohort was used to conduct external validation by calculating C-index and plot a 1-year calibration curve. As the cut-off date was December 31 2018, we validated the nomogram just in 1 year with the validation cohort. All potential variables were finally employed to draw Kaplan-Meier curve and to conduct a Cox proportional hazard regression to find the risk and protective factors of PSCC. P<0.05 of two-sided was considered statistically significant and the analysis was performed in R software (4.1.2, https://www.r-project.org/) using these packages: epiDisplay, glmnet, rms, survival, pec and ezcox.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The authors signed the Data-Use Agreement for the 1975–2018 SEER Research Data File. This study has been approved by the Ethics Committee of Beijing Chest Hospital affiliated to Capital Medical University (No. LW-2022-001) and individual consent for this retrospective analysis was waived.
Results
Different OS possibilities among PSC
The Kaplan-Meier plot using data from 2,764 patients showed five subtypes of PSC owned different OS (P<0.0001, Figure 2). The median OS time of pleomorphic carcinoma patients was 7 months [interquartile range (IQR), 2–27 months; range, 1–203 months]; the median OS time of giant cell carcinoma patients was 4 months (IQR, 1–13 months; range, 1–227 months); the median OS time of pulmonary blastoma patients was 28 months (IQR, 8–106 months; range, 1–226 months); the median OS time of carcinosarcoma patients was 7 months (IQR, 2–24 months; range, 1–219 months); and the median OS of PSCC patients was 4 months (IQR, 1–14 months; range, 1–218 months; Table 2, Table S2). Cox regression indicated compared to PSCC, giant cell carcinoma patients had shorter OS (HR, 1.14; 95% CI: 1.01–1.28) while pleomorphic carcinoma (HR, 0.70; 95% CI: 0.62–0.79), pulmonary blastoma (HR, 0.35; 95% CI: 0.25–0.51) and carcinosarcoma (HR, 0.81; 95% CI: 0.71–0.93) patients had longer OS (Figure 2).
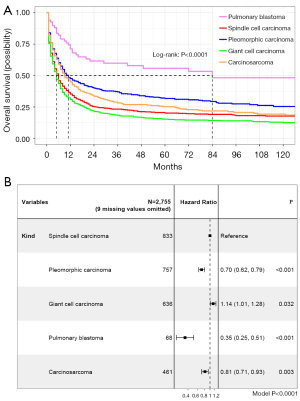
Table 2
| Variables | Median [IQR] (months) | Range (months) |
|---|---|---|
| All (N=833) | 4 [1, 14] | 1–218 |
| Surgery (N=216) | 18 [5, 72] | 1–218 |
| Chemotherapy (N=256) | 7 [3, 17] | 1–218 |
| Radiotherapy (N=260) | 5 [2, 14] | 1–218 |
OS, overall survival; PSCC, pulmonary spindle cell carcinoma; IQR, interquartile range.
Development and validation of nomogram
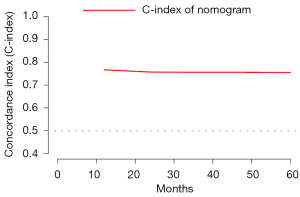
Using the primary cohort, we established a nomogram to predict PSCC patients OS probability in 1-, 3- and 5-year (Figure 3). The C-index of nomogram in the primary cohort was 0.79 (95% CI: 0.76–0.82). With 1,000 bootstrap samples, the C-index in 1-, 2-, 3-, 4- and 5-year were 0.77, 0.76, 0.76 0.76, 0.76, whose trend was plotted in Figure 4. Three calibration curves (Figure 5A-5C) for predicting the 1-, 3- and 5-year OS probability were plotted, indicating a good discrimination of the nomogram in internal validation. The C-index in the validation cohort was 0.76 (1 year OS probability) and the calibration curve showed a good discrimination (Figure 5D).


Survival analysis
Age, sex, race, site, grade, stage, radiotherapy, surgery, chemotherapy, lymphatic metastasis, size, distant metastasis, and history of malignancy were used to conduct a multivariate Cox proportional hazard regression using all primary and validation cohort data (N=247, with missing values of above variables omitted). Patients of older age (HR, 1.02; 95% CI: 1.01–1.04), larger size of neoplasm (HR, 1.01; 95% CI: 1.01–1.01), M1 distant metastasis (HR, 2.96; 95% CI: 2.17–4.04), N2 (HR, 2.55; 95% CI: 1.81–3.59) or N3 (HR, 2.99; 95% CI: 1.58–5.66), regional stages (HR, 2.11; 95% CI: 1.29–3.44) and distant stages (HR, 6.17; 95% CI: 3.83–9.94) had a lower OS possibility, while surgery (HR, 0.39; 95% CI: 0.28–0.53) and history of malignancy (HR, 0.68; 95% CI: 0.48–0.98) were protective factors for PSCC (Figure 6). The median OS of PSCC patients was 4 months (IQR, 1–14 months; range, 1–218 months, Table 2), and the Kaplan-Meier cure was plotted in Figure 7A. Different treatments might produce different therapeutic effects, surgery indicating a longer OS (Figure 7B-7D). The median OS of received surgery patients was 18 (IQR, 5–72 months; range, 1–218 months). The median OS of received chemotherapy patients was 7 (IQR, 3–17 months; range, 1–218 months). And the median OS of received radiotherapy patients was 5 (IQR, 2–14 months; range, 1–218 months) (Table 2). The OS probability of 1-, 2-, 3-, 4- and 5-year was 35.3% (95% CI: 32.0–38.9%), 25.2% (95% CI: 22.1–28.7%), 23.0% (estimated by life table, Table S3), 21.0% (estimated by life table), 20.1% (95% CI: 17.2–23.6%) (Table 3).
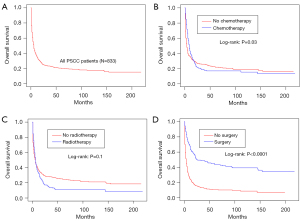
Table 3
Discussion
We first discuss the OS time, influential factors, develop and validate a predictive nomogram of PSCC patients using large samples’ data. By contrasting five subtypes from 2,764 patients’ data, we found the OS differed in PSC. Then we analyzed 833 PSCC patients and found older age, larger size, M1, N2, N3, regional stages, and distant stages meant a short OS time. Surgery was an effective treatment of PSCC instead of chemotherapy or radiotherapy. Radiotherapy had no effect on PSCC while patients treated with chemotherapy underwent a shorter OS in the long terms. The nomogram developed by us can offer an OS prediction of PSCC patients.
WHO classified PSC into five subtypes: pleomorphic carcinoma, pulmonary blastoma, giant cell carcinoma, carcinosarcoma, and spindle cell carcinoma (1). As a rare NSCLC, there were few studies on PSC and much fewer on PSCC. For there are few papers discussing the prognosis and treatment of PSCC, we compared our study with some PSCs.
Ung et al. reported the median size of PSC was about 6 cm, which is similar to our observation (the median of size 52.50 mm) (22). The 5-year OS in our observation is 20.1% (95% CI: 17.2–23.6%), which is similar to other reports (4,23,24). But there are studies reporting different OS, the deviation might result from the difference between PSCC and PSC (2,25). For PSC, smoking, N3, T3 and T4 is dangerous factors and surgery (median OS time 16.4 months; our observation was 18 months) has significant impact on survival (2,22,24), and the similar consequence was revealed by our study. But Ung et al. considered age was not a significant factor, which is different from our analysis. The imparity might result from the age range and the number of samples: they analyzed 93 patients whose ages’ median is 63 years (range, 40–85 years), and we observed 833 patients whose ages median is 73 years (range, 21–96 years) (22). But they did agree chemotherapy has no effective influence on survival either. Usually, PSC have a high resistance to chemotherapy whose stand point is the same with ours, but others reported a platinum-based combination chemotherapy might have influence on OS (4,15,22).
Domblides et al. indicated after treated with immune checkpoint inhibitors (ICIs) such like nivolumab (86.5% of cases), the objective response rate of 37 sarcomatoid carcinoma patients was 40.5% and disease control rate was 64.8% regardless of PD-L1 status, while the objective response rate of patients with PD-L1+ was 58.8% (26). Jiao et al. found received treatment with PD-1 inhibitor toripalimab, the tumor of an advanced PSC patient shrank and the response evaluation was partial response (PR) (27). As PSCs and pleomorphic carcinoma patients usually highly expressed PD-L1 (53–90%), immunotherapy might release huge potential in the therapy of PSC (11,28,29).
Above all, PSC patients have a poor prognosis and there are only limited therapy options. Surgery might be the better one on the OS time instead of chemotherapy or radiotherapy. Some studies argued C-MET inhibitor, targeted drugs, PD-1 or PD-L1 might be the potential therapy of PSC (5,10-14,30,31), but more studies related still needed.
This retrospective study is conducted using data from SEER database, which might bring out some selection bias and information bias. Patients of PSC are usually smoking but there is no more information about smoke status in SEER database (4,22,23). The sample size of this study is modest.
Conclusions
PSCC patients have a poor prognosis, using the nomogram developed by this study can predict their 1-, 3- and 5-year OS probability. The nomogram shows a good concordance and discrimination on both the primary and validation cohorts. Surgery is a better option for PSCC and more studies are necessary to find potential treatments like targeted therapy, PD-1 or PD-L1.
Acknowledgments
We are grateful for the help of the support team from The Surveillance, Epidemiology, and End Results at the National Cancer Institute.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-427/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-427/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The authors signed the Data-Use Agreement for the 1975–2018 SEER Research Data File. This study has been approved by the Ethics Committee of Beijing Chest Hospital affiliated to Capital Medical University (No. LW-2022-001) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 2007;84:973-80. [Crossref] [PubMed]
- Qi DJ, Liu B, Feng L, et al. Pulmonary spindle cell carcinoma with unusual morphology: A rare case report and review of the literature. Medicine (Baltimore) 2017;96:e7129. [Crossref] [PubMed]
- Weissferdt A. Pulmonary Sarcomatoid Carcinomas: A Review. Adv Anat Pathol 2018;25:304-13. [Crossref] [PubMed]
- Schrock AB, Li SD, Frampton GM, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol 2017;12:932-42. [Crossref] [PubMed]
- Fallet V, Saffroy R, Girard N, et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta™ Panel: exploring therapeutic targets. Ann Oncol 2015;26:1748-53. [Crossref] [PubMed]
- Rossi G, Nosseir S, Jocollé G, et al. Infarct-Like Spindle Cell Carcinoma of the Lung: Clinicopathologic, Immunohistochemical, and Molecular Analysis of 4 Cases. Int J Surg Pathol 2020;28:616-23. [Crossref] [PubMed]
- Morimoto M, Osaki T, Kodate M, et al. Spindle cell carcinoma of the lung. Gen Thorac Cardiovasc Surg 2011;59:129-32. [Crossref] [PubMed]
- Kida J, Kanaji N, Kishi S, et al. An Autopsy Case of Rapidly Progressing Spindle Cell Carcinoma of the Lung Accompanied with Intratumor Hemorrhage. Am J Case Rep 2015;16:805-10. [Crossref] [PubMed]
- Lee C, Usenko D, Frampton GM, et al. MET 14 Deletion in Sarcomatoid Non-Small-Cell Lung Cancer Detected by Next-Generation Sequencing and Successfully Treated with a MET Inhibitor. J Thorac Oncol 2015;10:e113-4. [Crossref] [PubMed]
- Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. [Crossref] [PubMed]
- Zou F, Xie G, Ma JA, et al. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: A case report. Oncol Lett 2015;9:2239-43. [Crossref] [PubMed]
- Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010;5:460-5. [Crossref] [PubMed]
- Sugano T, Mori M, Namba Y, et al. A case of sarcomatoid carcinoma of the lung successfully treated with carboplatin, paclitaxel and bevacizumab. Nihon Kokyuki Gakkai Zasshi 2011;49:304-8. [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Kontic M, Stojsic J, Stevic R, et al. Could spindle cell lung carcinoma be considered and treated as sarcoma, according to its clinical course, morphology, immunophenotype and genetic finding? Pathol Oncol Res 2013;19:129-33. [Crossref] [PubMed]
- LuLu X. Jian S. Concomitance of pulmonary spindle cell carcinoma and sclerosing pneumocytoma in a woman: A case report. Medicine (Baltimore) 2019;98:e18416. [Crossref] [PubMed]
- Matsui K, Kitagawa M. Spindle cell carcinoma of the lung. A clinicopathologic study of three cases. Cancer 1991;67:2361-7. [Crossref] [PubMed]
- Tonai K, Kitasato Y, Kawakami T, et al. Autopsy case of rapidly progressive pulmonary spindle cell carcinoma with multiple metastases to the brain and pancreas. Nihon Kokyuki Gakkai Zasshi 2009;47:828-32. [PubMed]
- Grosse A, Grosse C. Combined large cell neuroendocrine carcinoma and spindle cell carcinoma of the lung: Report of a rare entity presenting in fine needle aspiration. Cytopathology 2021;32:132-5. [Crossref] [PubMed]
- Mamanov M, Yener M, Yilmaz M, et al. Spindle cell carcinoma of the larynx following spindle cell pulmonary carcinoma: second primary or metastasis? J Craniofac Surg 2012;23:1935-6. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311-24. [Crossref] [PubMed]
- Gu L, Xu Y, Chen Z, et al. Clinical analysis of 95 cases of pulmonary sarcomatoid carcinoma. Biomed Pharmacother 2015;76:134-40. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Correa AM, et al. "Sarcomatoid" carcinomas of the lung: a clinicopathological study of 86 cases with a new perspective on tumor classification. Hum Pathol 2017;63:14-26. [Crossref] [PubMed]
- Domblides C, Leroy K, Monnet I, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol 2020;15:860-6. [Crossref] [PubMed]
- Jiao Y, Liu M, Luo N, et al. Successful treatment of advanced pulmonary sarcomatoid carcinoma with the PD-1 inhibitor toripalimab: A case report. Oral Oncol 2021;112:104992. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51-8. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Pelosi G, Gasparini P, Conte D, et al. Synergistic Activation upon MET and ALK Coamplification Sustains Targeted Therapy in Sarcomatoid Carcinoma, a Deadly Subtype of Lung Cancer. J Thorac Oncol 2016;11:718-28. [Crossref] [PubMed]



