
Durable response to crizotinib in an advanced lung adenocarcinoma patient harboring rare CD47-MET fusion: a case report
Introduction
MET gene, located on chromosome 7q21-31, encodes a receptor tyrosine kinase, activated by the binding of hepatocyte growth factor (HGF). Activating alterations in MET, achieved mainly via overexpression, amplification, mutations, or alternative splicing, have been discovered in non-small cell lung cancer (NSCLC). The activity of MET tyrosine kinase inhibitors (TKIs), such as crizotinib, capmatinib, tepotinib, and savolitinib, varies by MET mutation category (1,2). MET fusion, a rare type of structure rearrangement initially described in a few cases (3-5), has recently been reported in 0.26% of NSCLCs (6). Although sporadic cases have suggested the efficacy of crizotinib in NSCLC with MET fusion (3-5), the therapeutic relevance of this type of alteration remains largely uncovered and warrants further evidence. Herein, we presented a case of an advanced lung adenocarcinoma patient with CD47-MET fusion, who achieved a durable response of 8 months to crizotinib. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-141/rc).
Case presentation
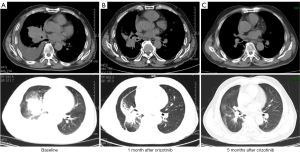
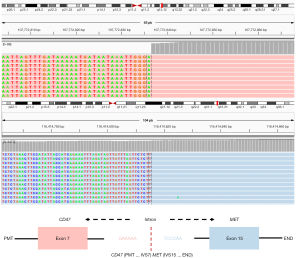
In April 2019, a 72-year-old male with a 10-year history of smoking presented to our hospital with coughing and blood-tinged sputum accompanied with chest distress and anhelation for 2 weeks. Physical examinations reported no abnormal symptoms except for diminished breath sounds. Imaging results were shown in Figure 1. Chest computed tomography (CT) scan revealed multiple scattered nodules on both lungs, atelectasis of right lower lobe, a mass near the hilum of lung, multiple enlarged lymph nodes on right lung hilum and mediastinum, and right pleural effusion (Figure 1A). No brain metastases were observed. CT-guided percutaneous biopsy was performed for histopathological examination, which indicated stage IV lung adenocarcinoma. Immunohistochemistry results showed positive expression of PD-L1, and the tumor proportion score (TPS) was 5%. The biopsied tissue was sent for next-generation sequencing (NGS) using a 520-gene panel (Burning Rock Biotech, Guangzhou, China) and a CD47 (EX7)-MET (EX15) rearrangement (Figure 2) was identified with an allele frequency (AF) of 9.75%. No other mutation was detected. The results also revealed a tumor mutational burden (TMB) of 2.4 mutations/Mb and a microsatellite stable status (MSS). Subsequently, crizotinib (250 mg/b.i.d. orally) was administrated starting from May 2019. After 1 month of treatment, the patient showed partial re-expansion of the collapsed right lower lobe, shrinkages of lymph node lesions, and reduction in right pleural effusion (Figure 1B). After 5 months of treatment, the patient remained as partial response (PR) (Figure 1C) with minimal side effects and eventually achieved a progression-free survival (PFS) of 8 months.
After progression on crizotinib, the patient was switched to cabozantinib in Feb 2020 with an initial dosage of 100 mg for 3 weeks, followed by a reduced dosage of 62.5 mg. A repeated chest CT performed in another hospital due to the COVID 19 pandemic in April 2020 (data was not available) revealed a significant shrinkage of the tumor lesion. Disease progressed in May 2020, and the patient was subsequently treated with a combination of pemetrexed and bevacizumab. A re-biopsy of the pulmonary lesion at cabozantinib progression was sent for NGS using a 168-gene panel (Burning Rock Biotech, Guangzhou, China), and results showed that the patients retained CD47-MET fusion (AF: 5.71%) and acquired a MET D1228E mutation (AF: 5.94%). No other mutation was identified. After two cycles of pemetrexed and bevacizumab treatment, the patient achieved a stable disease (data was not available). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The CD47-MET rearrangement identified in our case had breakpoints in the CD47 intron 7 and MET intron 14, which conferred the fusion of CD47 exon 7 to MET exon 15 (Figure 2). This fusion was first identified in a stage IA lung adenocarcinoma patient who underwent surgical resection (7). However, the therapeutic relevance of this fusion has not been reported. Our study provides the first clinical evidence for the efficacy of crizotinib in CD47-MET rearranged NSCLC, which is in line with previous case reports on responses of MET fusions to crizotinib, including HLA-DRB1-MET, MET–ATXN7L1, KIF5B-MET, STARD3NL-MET, and MET-UBE2H (3-5,8,9). The response duration reported in these studies ranged from 4 months to 12 months with a median duration of 8 months, consistent with the PFS of 8 months observed in our case.
Crizotinib, originally developed as a MET inhibitor, has now been approved by the FDA only for ALK or ROS1 rearranged NSCLC patients. However, numerous studies have revealed clinical benefits with crizotinib in patients harboring MET amplification or MET exon 14 skipping mutation (10,11). Our case further supports that MET fusion represents a novel molecular target for crizotinib in NSCLC. The utility of MET TKIs was not fully understood in MET fusion-positive cancers, especially in NSCLC. Besides some isolated cases that have shown responses to crizotinib in MET fusion-positive NSCLC and gliomas (5,8,12), some clinical trials are underway to assess the efficacy of MET TKIs in cancers including those with MET fusions. At present, a phase II trial (NCT01639508) is underway to evaluate the effectiveness of cabozantinib in advanced NSCLC patients, including those with MET fusions. The efficacy of tepotinib, FDA approved for application in metastatic NSCLC patients with the METex14 mutation, was assessed with or without other TKIs in MET-driven NSCLC (NCT04739358). The safety and efficacy of TPX-0022, a MET/CSF1R/SRC inhibitor, are being evaluated in patients harboring MET fusions in a phase 1 study (NCT03993873). Another phase I clinical trial (NCT02978261) of a c-Met inhibitor PLB1001 was conducted in patients with PTPRZ1-MET fusion gene-positive recurrent high-grade gliomas.
MET D1228E mutation was identified at disease progression on second-line cabozantinib. Acquired MET D1228N/H/V has been reported to mediate resistance to type I MET TKIs (including crizotinib) through impaired drug binding (13,14). Since the genomic profile at crizotinib progression was unknown in our case, MET D1228E is more likely to emerge as a resistance mutation to crizotinib. In vitro and preliminary clinical evidence revealed that MET D1228V/H retained sensitivity to the type II MET inhibitor cabozantinib, while D1228N might confer resistance to it (13,14). Interestingly, CD47-MET fusion and MET D1228E were present at similar frequencies (5.71% and 5.94%, respectively) in the re-biopsy of the pulmonary lesion at cabozantinib progression, suggesting that the two mutations might be on the same allele of the MET fusion gene. However, they were located in exon 15 and exon 19, respectively, and we can’t confirm it by DNA-based NGS. In our study, the response to cabozantinib was initially observed but failed to sustain. Both CD47-MET fusion and MET D1228E were retained at cabozantinib progression. Therefore, the responsiveness of CD47-MET fusion and MET D1228E to cabozantinib remains inconclusive and requires further exploration.
In conclusion, we described the first case for the efficacy of crizotinib in CD47-MET rearranged NSCLC and suggested MET D1228E emerging as a putative resistance mechanism. Our study highlights the therapeutic actionability of MET fusion and paves the way for establishing therapy for MET-rearranged NSCLC.
Acknowledgments
The authors thank the patient for agreeing to publish this report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-141/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-141/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo R, Luo J, Chang J, et al. MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat Rev Clin Oncol 2020;17:569-87. [Crossref] [PubMed]
- Terlecka P, Krawczyk P, Grenda A, et al. MET Gene Dysregulation as a Promising Therapeutic Target in Lung Cancer-A Review. J Pers Med 2021;11:1370. [Crossref] [PubMed]
- Cho JH, Ku BM, Sun JM, et al. KIF5B-MET Gene Rearrangement with Robust Antitumor Activity in Response to Crizotinib in Lung Adenocarcinoma. J Thorac Oncol 2018;13:e29-31. [Crossref] [PubMed]
- Zhu YC, Wang WX, Song ZB, et al. MET-UBE2H Fusion as a Novel Mechanism of Acquired EGFR Resistance in Lung Adenocarcinoma. J Thorac Oncol 2018;13:e202-4. [Crossref] [PubMed]
- Zhu YC, Wang WX, Xu CW, et al. Identification of a novel crizotinib-sensitive MET-ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol 2018;29:2392-3. [Crossref] [PubMed]
- Zhuo M, Liang Z, Yi Y, et al. Analysis of MET kinase domain rearrangement in NSCLC. Lung Cancer 2020;145:140-3. [Crossref] [PubMed]
- Pan Y, Zhang Y, Ye T, et al. Detection of Novel NRG1, EGFR, and MET Fusions in Lung Adenocarcinomas in the Chinese Population. J Thorac Oncol 2019;14:2003-8. [Crossref] [PubMed]
- Davies KD, Ng TL, Estrada-Bernal A, et al. Dramatic Response to Crizotinib in a Patient with Lung Cancer Positive for an HLA-DRB1-MET Gene Fusion. JCO Precis Oncol 2017; [Crossref] [PubMed]
- Plenker D, Bertrand M, de Langen AJ, et al. Structural Alterations of MET Trigger Response to MET Kinase Inhibition in Lung Adenocarcinoma Patients. Clin Cancer Res 2018;24:1337-43. [Crossref] [PubMed]
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- International Cancer Genome Consortium PedBrain Tumor Project. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med 2016;22:1314-20. [Crossref] [PubMed]
- Bahcall M, Sim T, Paweletz CP, et al. Acquired METD1228V Mutation and Resistance to MET Inhibition in Lung Cancer. Cancer Discov 2016;6:1334-41. [Crossref] [PubMed]
- Kang J, Chen HJ, Wang Z, et al. Osimertinib and Cabozantinib Combinatorial Therapy in an EGFR-Mutant Lung Adenocarcinoma Patient with Multiple MET Secondary-Site Mutations after Resistance to Crizotinib. J Thorac Oncol 2018;13:e49-53. [Crossref] [PubMed]


