
Severe side effects caused by parenteral nutrition therapy with fat emulsion (10%)/amino acids (15)/glucose (20%) injection: 2 case reports
Introduction
The body requires specific amounts of carbohydrates, protein, fat, vitamins, and minerals to maintain growth, healing, and vitality. For a variety of reasons, gastrointestinal function is not adequate to obtain necessary nutrients and fluid from food alone in some people. For instance, patients with malignant tumors present a high metabolic state and abnormal energy consumption, leading to malnutrition or cachexia. This could directly shorten the survival of patients and affect their quality of life. Around 31–87% of patients with malignant tumors have nutritional deficiencies, especially those with cholangiocarcinoma, liver cancer, gastric cancer, and other digestive system tumors (1,2). It is reported that 5% of cancer patients are in a state of malnutrition at the time of diagnosis, the incidence of malnutrition in patients who die of malignant tumors is almost 100%, and the prognosis of malnourished patients is significantly worse than that of well-nourished patients (3,4).
Nutritional support can significantly improve the metabolic status of patients and enhance the tolerance threshold of chemotherapy, which is contributed to a higher success rate of treatment and lower incidence of side effects of chemotherapy (5). Parenteral nutrition (PN) refers to a method of providing nutrients via intravenous injection, instead of the gastrointestinal tract. The liquid of PN is usually in the form of a mixture of several nutritional ingredients, such as fat emulsion, amino acids, and glucose. The application of PN may incur some side effects, including phlebitis, cholestasis, chills, nausea, vomiting, and Wernicke encephalopathy, according to the medicine instructions (6,7). Currently, the PN mixture of fat emulsion, amino acids, and glucose is widely applied in clinic; however, the related adverse reactions have been rarely reported.
Here, we report the cases of 2 patients who received fat emulsion (10%)/amino acids (15)/glucose (20%) injection and experienced rare severe side effects, hoping to provide new insight into PN application and side-effects recognition for physicians. It is crucial to inquire about the patient’s allergy history, and formulate an individualized PN administration strategy based on their laboratory testing. Once the suspected symptoms of side effects caused by PN appear, instant judgment and treatment should be performed to ensure the safety of patients. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1442/rc).
Case presentation
Case 1
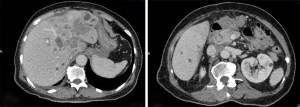
The first case was female, 69 years old, with a height of 153 cm, weight 51 kg, and body mass index (BMI) 21.8 kg/m2. She was admitted to the hospital diagnosed with cholangiocarcinoma T2N1MX IIIB on 12 January 2021. Magnetic resonance imaging (MRI) examination revealed several scattered low-density signals in the liver and bile duct region (Figure 1). The patient had no previous history of drug or food allergy. On 27 January 2021, her manifestations of nausea, abdominal distension and pain were worse than before, and food intake had decreased by 40%, with obvious weight loss. The Nutritional Risk Screening (NRS) 2002 score was 4, suggesting the risk of malnutrition (8). This patient received 1,000 mL fat emulsion (10%)/amino acids (15)/glucose (20%) injection (Duoyue, Kelun Pharmaceutical Co., Ltd., Sichuan, China; approval number: H20183272, batch No.: P20121103), 1 g potassium chloride injection (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan; batch No.: OG99K3), 1 g concentrated sodium chloride injection (Huarun Shuanghe Limin Pharmaceutical Co., Ltd., Jinan, China; batch No.: 20100544), and multiple microelement injection (II) 10 mL (Meida Kangjiale Pharmaceutical Co., Ltd., Sichuan, China; batch No.: 20080672). These solutions were pumped into a 3-liter bag and intravenously injected into the patient, once a day.
On 2 February, the patient received the PN solution mentioned above at 2:30 pm; at 6:30 pm, she suddenly experienced chills and aversion to cold, there was no aggravation of chest pain or breathing obstruction, no dyspnea, and no palpitation. Auscultation detected the presence of rough respiration in both lungs, with some dry rales. The heart rate was 100/min with regular heart rhythm, and no pathological murmur was heard in each valve. The blood pressure was 175/100 mmHg.
The injection of the PN solution was stopped immediately, the infusion set was replaced, and physiological saline was dropped intravenously. She received oxygen inhalation, and 5 mg dexamethasone and 25 mg promethazine hydrochloride were injected intravenously and intramuscularly, respectively. We kept the patient warm and her symptoms improved 30 minutes later. On 4 February, the above PN solution was repeatedly given, and no side effects occurred until discharge.
Case 2
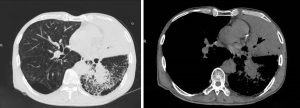
The second case was male, 69 years old, with a height of 175 cm, weight 73 kg, and BMI 23.8 kg/m2. He was admitted to the hospital diagnosed with lung cancer (differentiated squamous cell carcinoma in the lower lobe of the right lung, stage T4NxM1 IV), and secondary hepatic malignant tumor on 13 February 2021, and the relevant MRI examination is shown in Figure 2. The patient had no previous history of food or drug allergy, but had a history of smoking and drinking for 40 years. Since the onset of the disease, he had lost about 10 kg of body weight and had a poor diet. This patient received 1,000 mL fat emulsion (10%)/amino acids (15)/glucose (20%) injection once a day.
At 4:00 pm on 18 February, the patient was given intravenous infusion of fat emulsion (10%)/ amino acid (15)/glucose (20%) (Duoyue). At 5:10 pm, the patient presented the aggravation of chest pain and breath obstruction, chills, cyanosis, and wheeze. Physical examination showed that the breath sounds were rough in both lungs, with a small amount of wheezing, although no moist rale was heard. The heart rate was 120/min, with regular heart rhythm, and pulmonary embolism was excluded.
The injection was stopped immediately, and 40 mg methylprednisolone sodium succinate was given by intravenous injection. Electrocardiograph monitoring showed the oxyhemoglobin saturation was 80%, the blood pressure was 220/113 mmHg, and the heart rate was 116/min. The patient’s symptoms improved 10 minutes later. On 21 February, the patient was given a 1,000 mL intravenous infusion of fat emulsion (10%)/amino acids (15)/glucose (20%) injection. At this time, we slowed down the dropping speed of the injection and the patient was under close monitoring. The above symptoms of side effects did not occur again.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Here, we have reported the cases of 2 patients who received fat emulsion (10%)/amino acids (15)/glucose (20%) injection and had severe side effects. We have described the details of clinical manifestations and corresponding treatment approaches, in the hope of strengthening the medicine perception in the clinic application.
Both cases did not have a history of drug allergy. Patient 1 presented symptoms of chills and aversion to cold after 7 days of fat emulsion (10%)/amino acids (15)/glucose (20%) injection. After 1 day of withdrawal, the patient recovered and did not experience similar side effects at the second application. Patient 2 had chest tightness, chills, cyanosis, and wheezing symptoms with a 6-day application of fat emulsion (10%)/amino acids (15)/glucose (20%) injection. It is recorded in the medicine specification that fat emulsion (10%)/amino acids (15)/glucose (20%) may cause some side effects such as chest tightness and chills, but no cyanosis was reported. After a 2-day intermediate rest, we slowed down the dropping speed of the injection this time to avoid the possible side effects induced by rapid intravenous drip. The patient was under close monitoring and the above symptoms of side effects did not occur again.
We carefully read some studies about the potential side effects aroused by fat emulsion (10%)/amino acids (15)/glucose (20%) injection based on the search of China National Knowledge Infrastructure (CNKI), involving the drug-induced liver injury, cholestasis, phlebitis, Wernicke’s encephalopathy, and hypoglycemia (9-14); however, no study related to cyanosis had been reported. Besides, we excluded the original disease which may have led to the side effects in these two patients, and after stopping and then resuming the medicine, no further adverse reaction was reported. According to the Karch and Lasagna assessment methods (15), we hypothesized that the chills, chest tightness, and cyanosis in these 2 patients may be caused by the fat emulsion (10%)/amino acids (15)/glucose (20%) injection.
We further analyzed the possible reason for side effects induced by fat emulsion (10%)/amino acids (15)/glucose (20%) injection. Firstly, we considered whether these symptoms belonged to “fat overload syndrome” (16). These two cases were both elderly patients with tumors, and their metabolic rates were relatively slow, which may lead to the issue of fat overload. Fat particles can deposit on the lungs, resulting in the decrease of pulmonary diffuse ability, even the presence of fat embolism. We reviewed the examination data of the two patients, and found they had elevated aspartate aminotransferase and D-dimer before injection, and these indexes showed no significant change when the adverse reactions occurred. Arterial blood gas analysis showed no hypoxia and hypocapnia, and no abnormalities of electrocardiograph and lung computed tomography (CT) were detected, which did not conform to the clinical manifestations of fat overload syndrome.
The second hypothesis we considered was the infusion speed. The maximum infusion rate of fat emulsion (10%)/amino acids (15)/glucose (20%) was 3 mL/kg/h. Both patients were elderly patients with the potential risk of cardiovascular events, and an excessive infusion speed may lead to congestive heart failure, dyspnea, and hypoxemia caused by acute pulmonary edema. After confirming with the nursing staff, the infusion speed was maintained at 50–100 mL/h in these two patients, which was far below the maximum infusion speed. Besides, we also identified the quality of medicine by communicating with the manufacturers about the materials and inspection of this batch of fat emulsion (10%)/amino acids (15)/glucose (20%), and no abnormality was found. Therefore, we indicated that the severe side effects in these two patients may have been caused by the fat emulsion (10%)/amino acids (15)/glucose (20%) injection.
Conclusions
Fat emulsion (10%)/amino acids (15)/glucose (20%) injection is a multicavity-bag standardized PN formula, which is suitable for patients with gastrointestinal insufficiency requiring PN support, especially for patients with advanced tumors. It is widely utilized in clinical practice, but few side effects have been reported. Here, we have reported the cases of two elderly tumor patients who received fat emulsion (10%)/amino acids (15)/glucose (20%) injection and subsequently experienced severe side effects, and the underlying mechanism is discussed.
In summary, physicians should inquire thoroughly about the patient’s allergy history, and formulate an individualized PN administration plan, for which central intravenous infusion is recommended. There is a need to strengthen the management of infusion speed and clinical medication, thus ensuring the safety of medicine application.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1442/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1442/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr 2018;72:1255-9. [Crossref] [PubMed]
- Kadakia KC, Symanowski JT, Aktas A, et al. Malnutrition risk at solid tumor diagnosis: the malnutrition screening tool in a large US cancer institute. Support Care Cancer 2022;30:2237-44. [Crossref] [PubMed]
- Sutton EH, Plyta M, Fragkos K, et al. Pre-treatment sarcopenic assessments as a prognostic factor for gynaecology cancer outcomes: systematic review and meta-analysis. Eur J Clin Nutr 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Meza-Valderrama D, Marco E, Dávalos-Yerovi V, et al. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021;13:761. [Crossref] [PubMed]
- Pang NQ, Tan YX, Samuel M, et al. Multimodal prehabilitation in older adults before major abdominal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Fedeli P, Justin Davies R, Cirocchi R, et al. Total parenteral nutrition-induced Wernicke's encephalopathy after oncologic gastrointestinal surgery. Open Med (Wars) 2020;15:709-13. [Crossref] [PubMed]
- Meyerson C, Naini BV. Something old, something new: liver injury associated with total parenteral nutrition therapy and immune checkpoint inhibitors. Hum Pathol 2020;96:39-47. [Crossref] [PubMed]
- Molfino A, Imbimbo G, Laviano A. Current Screening Methods for the Risk or Presence of Malnutrition in Cancer Patients. Cancer Manag Res 2022;14:561-7. [Crossref] [PubMed]
- Wang W, Tang M. Liver injury and cholestasis due to concomitant use of alanyl glutamine injection and fat emulsion, amino acids (17) and glucose (11%) injection. Adverse Drug Reactions Journal 2019;21:227-8.
- Guan L, Li X, Shi Y. Fat emulsion,amino acids (17) and glucose (11%) injection (1440 mL) induces drug-induced liver injury: a case report. Chinese Journal of Clinical Rational Drug Use 2018;11:116-17.
- Luo M, Cai X, Ye F, et al. Wernicke encephalopathy induced by long-term administration of fat emulsion, amino acids (17) and glucose (11%) injection in stroke patients. Herald of Medicine 2015;34:822-3.
- Hu C, Zhao P. Analysis of a case of phlebitis caused by fat emulsion, amino acids and glucose injection. Anti-Infection Pharmacy 2014;11:75-6.
- Huang B, Shi F, Tan H, et al. Adverse reaction of fat emulsion, amino acids (17) and glucose (11%) injection: a case report. Central South Pharmacy 2013;11:718.
- Zhang X, Guo B, Liu F, et al. Hypoglycemia induced by fat emulsion (10%), amino acids (15) and glucose (20%) injection: 2 cases report. Adverse Drug Reactions Journal 2019;21:229-30.
- Ribeiro A, Lima S, Zampieri ME, et al. Filling quality of the reports of adverse drug reactions received at the Pharmacovigilance Centre of São Paulo (Brazil): missing information hinders the analysis of suspected associations. Expert Opin Drug Saf 2017;16:1329-34. [Crossref] [PubMed]
- Man Z, Yan S. Fat overloading syndrome following intravenous infusion of fat emulsion in two elderly patients. Adverse Drug Reactions Journal 2008;10:167,172.


