
Incidence, prognostic factors and survival in bladder cancer patients: a population-based study
Introduction
Bladder cancer (BC) is the 10th most common cancer in the world and the 13th leading cause of cancer-related death (1), with nearly 549,000 new cases and 200,000 deaths each year (2).
Previous studies have shown that multiple factors, such as sex, age, smoking, and race, can affect the survival probability of BC (3-5). Study shows that the incidence of BC is three to four times higher in men than in women (6). The incidence of BC increases with age: more than 90% of patients are over 55 years old, with an average age of approximately 73 years (1). Whites are more likely to develop BC than people of other races (7). Survival probability is a prediction of the likelihood that a patient will continue to survive (8-10). Because each survival probability is a prediction based on finite conditions, the survival probability can only be used as a reference, and the survival probability cannot provide a definite answer.
The purpose of this study was to understand the incidence, prognostic factors, and survival trends in BC patients. This study was based on a larger population derived from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute. We also characterized independent prognostic factors associated with BC and sought to build prognostic nomograms that could assist clinicians in evaluating prognosis. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-46/rc).
Methods
Data source
The data for this study were obtained retrospectively from the SEER database. To extract data, select cases, and define variables, SEER*Stat (version 8.3.9.2) was used. The case listing was created using the incidence-SEER 18 Registers Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975-2016 varying). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patient identification
Patients diagnosed with malignant BC by histology between 2010 and 2015 were included in this study. Table 1 shows the inclusion and exclusion criteria for this study.
Table 1
| Inclusion criteria |
| Patients diagnosed with malignant bladder cancer |
| The histologic type of patients should be available |
| The survival time and vital status should be available |
| Clinicopathological information for the age at diagnosis, race, surgery, tumor stage and other baseline information should be available |
| Exclusion criteria |
| Patients with bladder cancer found during an autopsy or on a death certificate |
| Unknown ethnicity |
| Unknown survival time |
| Unknown AJCC-stage |
| Unknown summary stage |
| Unknown surgical status |
| Unknown TNM-T/N/M stage |
| Unknown age |
| Unknown vital status |
AJCC, American Joint Committee on Cancer; TNM, tumor, node, metastasis.
Variables from the selected cohort included sex, race, age at diagnosis, histologic type, TNM-T, TNM-N, TNM-M, AJCC stage, surgery of primary site, bone metastasis, brain metastasis, liver metastasis, lung metastasis, summary stage, survival months, and vital status. The main endpoint was overall survival (OS).
The age at diagnosis was divided into five subgroups: <50, 51–60, 61–70, 71–80, and >80 years. We classified the histological type into transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma, neuroendocrine carcinoma and other epithelial tumors of the bladder using the ICD-0-3 morphological code.
Statistical analysis
The incidence rates of BC were calculated per 100,000 person-years and age-adjusted to the 2,000 US Standard Population [19 age groups, United States Bureau of the Census, Current Population Reports, Publication 25-1130 (Census P25-1130)] using SEER*Stat (version 8.3.9.2). Annual percentage changes (APCs) were calculated using the weighted least squares method.
Estimated OS was calculated with the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors associated with OS in BC patients.
All statistical analyses were performed in R (Version 4.1.1, R Foundation; R packages: rms, survival, survminer, ggplot2). Statistical significance was set at a two-sided P value <0.05.
Nomogram construction
R 4.1.1 was used to construct a nomogram based on the results of multivariate analysis of the Cox proportional hazards model. The maximum score for each variable was set to 100. We also built calibration curves to identify whether the predicted and actual survival were in agreement.
Results
Baseline characteristics
A total of 95,329 eligible BC patients from 2010 to 2015 were included in this study. Table 2 shows the comparison of baseline characteristics and chi-square test results for patients with BC clustered by age. Of all patients, 4,139 (4.30%) patients were aged less than 51 years, 12,536 (13.20%) patients were aged 51–60 years, 25,979 (27.30%) patients were aged 61–70 years, 28,868 (30.20%) patients were aged 71–80 years and 23,807 (25.00%) patients were aged over 80 years. According to the chi-square test, sex (P<0.001), race (P<0.001), diagnosis year (P<0.001), histologic type (P<0.001), TNM-T (P<0.001), TNM-N (P<0.001), TNM-M (P<0.001), AJCC stage (P<0.001), surgery (P<0.001), bone metastasis (P<0.001), brain metastasis (P=0.045), and summary stage (P<0.001) were all factors that were significantly different among age groups, except for liver metastasis (P=0.851) and lung metastasis (P=0.101).
Table 2
| Factors | ALL, n (%) | Age group, n (%) | P value | ||||
|---|---|---|---|---|---|---|---|
| <51 years | 51–60 years | 61–70 years | 71–80 years | >80 years | |||
| Total | 95,329 (100.00) | 4,139 (4.30) | 12,536 (13.20) | 25,979 (27.30) | 28,868 (30.20) | 23,807 (25.00) | |
| Gender | <0.001 | ||||||
| Male | 73,059 (76.64) | 3,072 (4.20) | 96,006 (13.10) | 20,236 (27.80) | 22,515 (30.80) | 17,630 (24.10) | |
| Female | 22,270 (23.36) | 1,067 (4.80) | 2,930 (13.20) | 5,743 (25.80) | 6,353 (28.50) | 6,177 (27.70) | |
| Race | <0.001 | ||||||
| White | 85,577 (89.77) | 3,593 (4.20) | 10,951 (12.70) | 23,260 (27.20) | 26,071 (30.50) | 21,702 (25.40) | |
| Black | 5,443 (5.71) | 342 (6.30) | 987 (18.10) | 1,615 (29.70) | 1,497 (27.50) | 1,002 (18.40) | |
| API | 3,997 (4.19) | 178 (4.50) | 534 (13.40) | 1,014 (25.40) | 1,222 (30.50) | 1,049 (26.20) | |
| AIAN | 312 (0.33) | 26 (8.30) | 64 (20.50) | 90 (28.80) | 78 (25.00) | 54 (17.30) | |
| Diagnosis year | <0.001 | ||||||
| 2010 | 15,561 (16.32) | 740 (4.80) | 2,113 (13.50) | 4,174 (26.80) | 4,667 (30.00) | 3,867 (24.90) | |
| 2011 | 15,543 (16.30) | 731 (4.70) | 2,034 (13.10) | 4,214 (27.10) | 4,636 (29.80) | 3,928 (25.30) | |
| 2012 | 15,994 (16.78) | 720 (4.50) | 2,068 (12.90) | 4,336 (27.10) | 4,828 (30.20) | 4,042 (25.30) | |
| 2013 | 15,822 (16.60) | 674 (4.30) | 2,027 (12.80) | 4,418 (27.90) | 4,724 (29.90) | 3,979 (25.10) | |
| 2014 | 16,174 (16.97) | 631 (3.90) | 2,195 (13.60) | 4,395 (27.20) | 4,916 (30.30) | 4,037 (25.00) | |
| 2015 | 16,235 (17.03) | 643 (4.00) | 2,099 (12.90) | 4,442 (27.30) | 5,097 (31.40) | 3,954 (24.40) | |
| Histologic type | <0.001 | ||||||
| Tcc | 91,963 (96.47) | 3,900 (4.20) | 12,049 (13.10) | 25,147 (27.30) | 27,915 (30.40) | 22,952 (25.00) | |
| Scc | 1,265 (1.33) | 82 (6.50) | 182 (14.40) | 279 (22.10) | 360 (28.50) | 362 (28.50) | |
| Ac | 845 (0.89) | 121 (14.30) | 174 (20.60) | 223 (26.40) | 185 (21.90) | 142 (16.80) | |
| Nec | 788 (0.83) | 20 (2.50) | 86 (10.90) | 209 (26.60) | 247 (31.30) | 226 (28.70) | |
| Oet | 468 (0.49) | 16 (3.40) | 45 (9.60) | 121 (25.90) | 161 (34.40) | 125 (26.70) | |
| TNM-T | <0.001 | ||||||
| Ta | 46,421 (48.70) | 2,444 (5.30) | 6,579 (14.10) | 13,257 (28.60) | 13,991 (30.10) | 10,150 (21.90) | |
| Tis | 4,052 (4.25) | 131 (3.20) | 468 (11.50) | 1,103 (27.20) | 1,350 (33.40) | 100 (24.70) | |
| T0 | 1 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.00) | 0 (0.00) | 0 (0.00) | |
| T1 | 23,616 (24.77) | 776 (3.30) | 2,774 (11.70) | 6,161 (26.10) | 7,248 (30.70) | 6,657 (28.20) | |
| T2 | 14,382 (15.09) | 427 (3.00) | 1,664 (11.50) | 3,478 (24.20) | 4,154 (28.90) | 4,659 (32.40) | |
| T3 | 3,977 (4.17) | 219 (5.50) | 607 (15.30) | 1,192 (30.00) | 1,252 (31.40) | 707 (17.80) | |
| T4 | 2,880 (3.02) | 142 (4.90) | 444 (15.40) | 787 (27.30) | 873 (30.30) | 634 (22.00) | |
| TNM-N | <0.001 | ||||||
| N0 | 91,377 (95.85) | 3,875 (4.20) | 11,751 (12.90) | 24,772 (27.10) | 27,756 (30.40) | 23,223 (25.40) | |
| N1 | 1,544 (1.62) | 105 (6.80) | 295 (19.10) | 487 (31.50) | 426 (27.60) | 231 (15.00) | |
| N2 | 1,938 (2.03) | 129 (6.70) | 400 (20.60) | 569 (29.30) | 544 (28.10) | 296 (15.30) | |
| N3 | 470 (0.49) | 60 (6.40) | 90 (19.10) | 151 (32.10) | 142 (30.20) | 57 (12.10) | |
| TNM-M | <0.001 | ||||||
| M0 | 92,977 (97.53) | 4,016 (4.30) | 12,170 (13.10) | 25,376 (27.30) | 28,161 (30.30) | 23,254 (25.00) | |
| M1 | 2,352 (2.47) | 123 (5.20) | 366 (15.60) | 603 (25.60) | 707 (30.10) | 553 (23.50) | |
| AJCC stage | <0.001 | ||||||
| Stage 0a | 46,421 (48.70) | 2,444 (5.30) | 6,579 (14.10) | 13,257 (28.60) | 13,991 (30.10) | 10,150 (21.90) | |
| Stage 0is | 4,052 (4.25) | 131 (3.20) | 468 (11.60) | 1,103 (27.20) | 1,350 (33.30) | 1,000 (24.70) | |
| Stage I | 23,054 (24.18) | 737 (3.20) | 2,675 (11.60) | 6,025 (26.10) | 7,093 (30.80) | 6,524 (28.30) | |
| Stage II | 12,275 (12.88) | 335 (2.70) | 1,309 (10.70) | 2,886 (23.60) | 3,541 (28.80) | 4,204 (34.20) | |
| Stage III | 3,896 (4.09) | 164 (4.20) | 501 (12.90) | 1,080 (27.70) | 1,253 (32.20) | 898 (23.00) | |
| Stage IV | 5,631 (5.91) | 328 (5.80) | 1,007 (17.80) | 1,628 (28.90) | 1,640 (29.20) | 1,031 (18.30) | |
| Surgery | <0.001 | ||||||
| TURBT | 84,713 (88.86) | 3,523 (4.20) | 10,656 (12.60) | 22,477 (26.50) | 25,568 (30.20) | 22,489 (26.50) | |
| PC | 1,265 (1.33) | 95 (7.50) | 202 (16.00) | 327 (25.80) | 359 (28.40) | 282 (22.30) | |
| RC | 9,351 (9.81) | 521 (5.60) | 1,678 (17.80) | 3,175 (34.00) | 2,941 (31.50) | 1,036 (11.10) | |
| Metastasis | |||||||
| Bone | 94,491 (99.12) | 4096 (4.30) | 12,378 (13.10) | 25,760 (27.30) | 28,625 (30.30) | 23,632 (25.00) | <0.001 |
| Brain | 95,266 (99.93) | 4135 (4.30) | 12,525 (13.10) | 25,954 (27.30) | 28,853 (30.30) | 23,799 (25.00) | 0.045 |
| Liver | 94,852 (99.50) | 4115 (4.30) | 12,468 (13.10) | 25,856 (27.30) | 28,752 (30.30) | 23,688 (25.00) | 0.851 |
| Lung | 94,552 (99.18) | 4,100 (4.30) | 12,419 (13.10) | 25,798 (27.30) | 28,627 (30.30) | 13,608 (25.00) | 0.101 |
| Summary stage | <0.001 | ||||||
| In situ | 49,517 (51.94) | 2,535 (5.10) | 6,932 (14.00) | 14,111 (28.50) | 15,056 (30.40) | 10,883 (22.00) | |
| Localized | 35,803 (37.56) | 1,094 (3.10) | 4,047 (11.30) | 9,065 (25.30) | 10,767 (30.10) | 10,830 (30.20) | |
| Regional | 6,967 (7.31) | 343 (4.90) | 1,064 (15.30) | 1,992 (28.60) | 2,140 (30.70) | 1,428 (20.50) | |
| Distant | 3,042 (3.19) | 167 (5.50) | 493 (16.20) | 811 (26.60) | 905 (29.80) | 666 (21.90) | |
AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; TURBT, transurethral resection of bladder tumor; PC, partial cystectomy; RC, radical cystectomy; Tcc, transitional cell carcinoma; Scc, squamous cell carcinoma; Ac, adenocarcinoma; Nec, neuroendocrine carcinoma; Oet, other epithelial tumors.
Males accounted for approximately 70% of the patients in each group. Patients over 61 years old accounted for approximately 80% of the sample in each group. Based on TNM stage, summary stage and AJCC-stage system, most patients were in the early stage of cancer (in situ, localized, AJCC-stage 0a, AJCC-stage 0is, AJCC-stage I, AJCC-stage II, TaN0M0 and T1N0M0); therefore, more than 80% of patients underwent transurethral resection of bladder tumor (TURBT). According to histological type, transitional cell carcinoma accounted for more than 90% of patients in each group. The incidence of brain metastases was the lowest compared to other distant organ metastases.
Cox proportional hazards model
Univariate and multivariate risk analyses of OS are shown in Table 3. Univariate risk analyses revealed that sex, age, histologic type, race, TNM stage, AJCC stage, surgery, tumor metastasis and summary stage were significant prognostic factors of OS. Variables in univariate risk analyses with a P value of less than 0.01 were included in multivariate risk analyses. The results indicated that age, histologic type, race, TNM stage, AJCC stage, surgery, tumor metastasis and summary stage were independent prognostic factors for OS.
Table 3
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | <0.001 | ||||
| Male | Reference | Reference | |||
| Female | 1.070 (1.043–1.098) | <0.001 | 0.981 (0.956–1.007) | 0.154 | |
| Age | <0.001 | ||||
| 0–50 years | Reference | Reference | |||
| 51–60 years | 1.391 (1.268–1.527) | <0.001 | 1.330 (1.212–1.460) | <0.001 | |
| 61–70 years | 1.738 (1.593–1.897) | <0.001 | 1.734 (1.589–1.892) | <0.001 | |
| 71–80 years | 2.830 (2.597–3.083) | <0.001 | 2.748 (2.612–3.102) | <0.001 | |
| >81 years | 5.814 (5.340–6.330) | <0.001 | 5.693 (5.226–6.202) | <0.001 | |
| Histologic type | <0.001 | ||||
| Tcc | Reference | Reference | |||
| Scc | 3.056 (2.849–3.278) | <0.001 | 1.821 (1.696–1.956) | <0.001 | |
| Ac | 1.804 (1.638–1.987) | <0.001 | 1.097 (0.994–1.211) | 0.067 | |
| Nec | 3.718 (3.420–4.043) | <0.001 | 1.244 (1.142–1.356) | <0.001 | |
| Oet | 1.548 (1.352–1.773) | <0.001 | 1.201 (1.046–1.379) | 0.009 | |
| Race | <0.001 | ||||
| White | Reference | Reference | |||
| Black | 1.327 (1.270–1.386) | <0.001 | 1.270 (1.215–1.328) | <0.001 | |
| API | 0.900 (0.849–0.955) | <0.001 | 0.844 (0.796–0.896) | <0.001 | |
| AIAN | 1.170 (0.970–1.413) | 0.101 | 1.117 (0.926–1.349) | 0.248 | |
| TNM-T | <0.001 | ||||
| Ta | Reference | Reference | |||
| Tis | 1.405 (1.319–1.497) | <0.001 | 1.255 (1.166–1.351) | <0.001 | |
| T0* | – | – | – | – | |
| T1 | 1.965 (1.907–2.025) | <0.001 | 5.729 (4.571–7.181) | <0.001 | |
| T2 | 4.949 (4.805–5.098) | <0.001 | 6.156 (4.988–7.599) | <0.001 | |
| T3 | 4.784 (4.574–5.004) | <0.001 | 8.059 (6.520–9.962) | <0.001 | |
| T4 | 8.483 (8.098–8.888) | <0.001 | 9.524 (7.703–11.774) | <0.001 | |
| TNM-N | <0.001 | ||||
| N0 | Reference | Reference | |||
| N1 | 3.400 (3.197–3.615) | <0.001 | 0.953 (0.849–1.030) | 0.172 | |
| N2 | 4.876 (4.632–5.134) | <0.001 | 1.269 (1.163–1.384) | <0.001 | |
| N3 | 5.368 (4.850–5.941) | <0.001 | 1.063 (0.939–1.204) | 0.332 | |
| TNM-M | <0.001 | ||||
| M0 | Reference | Reference | |||
| M1 | 8.824 (8.438–9.228) | <0.001 | 0.996 (0.884–1.123) | 0.951 | |
| AJCC stage | <0.001 | ||||
| Stage 0a | Reference | Reference | |||
| Stage 0is | 1.405 (1.319–1.497) | <0.001 | – | – | |
| Stage I | 1.872 (1.815–1.930) | <0.001 | 0.257 (0.214–0.307) | <0.001 | |
| Stage II | 4.434 (4.297–4.575) | <0.001 | 0.640 (0.546–0.750) | <0.001 | |
| Stage III | 4.569 (4.364–4.783) | <0.001 | 0.564 (0.509–0.625) | <0.001 | |
| Stage IV | 9.549 (9.209–9.902) | <0.001 | – | – | |
| Surgery | <0.001 | ||||
| TURBT | Reference | Reference | |||
| PC | 1.201 (1.097–1.315) | <0.001 | 0.519 (0.472–0.571) | <0.001 | |
| RC | 1.595 (1.544–1.648) | <0.001 | 0.528 (0.507–0.551) | <0.001 | |
| Tumor metastasis | |||||
| Bone | <0.001 | ||||
| No | Reference | Reference | |||
| Yes | 9.716 (9.047–10.435) | <0.001 | 1.448 (1.324–1.584) | <0.001 | |
| Brain | <0.001 | ||||
| No | Reference | Reference | |||
| Yes | 12.727 (9.936–16.301) | <0.001 | 1.930 (1.501–2.482) | <0.001 | |
| Liver | <0.001 | ||||
| No | Reference | Reference | |||
| Yes | 12.104 (11.298–13.619) | <0.001 | 1.638 (1.473–1.821) | <0.001 | |
| Lung | <0.001 | ||||
| No | Reference | Reference | |||
| Yes | 9.466 (8.788–10.196) | <0.001 | 1.193 (1.088–1.307) | <0.001 | |
| Summary stage | <0.001 | ||||
| In situ | Reference | Reference | |||
| Localized | 2.492 (2.429–2.557) | <0.001 | 1.212 (1.059–1.387) | 0.005 | |
| Regional | 4.972 (4.797–5.153) | <0.001 | 1.412 (1.175–1.697) | <0.001 | |
| Distant | 14.457 (13.843–15.098) | <0.001 | 2.057 (1.653–2.558) | <0.001 | |
*, there was only one T0 stage patient, the result is not informative. AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; Tcc, transitional cell carcinoma; Scc, squamous cell carcinoma; Ac, adenocarcinoma; Nec, neuroendocrine carcinoma; Oet, other epithelial tumors; TURBT, transurethral resection of bladder tumor; PC, partial cystectomy; RC, radical cystectomy.
Incidence of BC
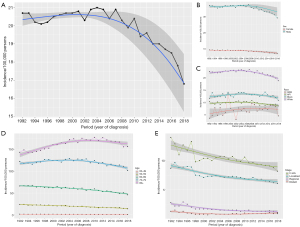
The trend in the incidence of BC has decreased since 1992, with an APC of −0.59 (95% CI: −0.79 to −0.39, P<0.05) (Figure 1A). This trend was more remarkable among male patients (Figure 1B), white patients (Figure 1C), patients aged less than 70 years (Figure 1D) and patients with in situ and localized tumor stage (Figure 1E). The overall annual age-adjusted incidences of BC from 1992 to 2018 were 20.7, 20.2, 20.1, 20.2, 20.5, 20.6, 20.7, 20.7, 0.8, 20.3, 20.9, 21, 20.9, 20.4, 20.9, 20.3, 19.9, 20.4, 19.4, 19.6, 19, 19, 18.7, 18.5, 17.8, and 16.8/100,000 persons, respectively.
Kaplan-Meier survival analysis
To evaluate the impact of different factors on the OS of BC patients, Kaplan-Meier survival analysis was performed in all patients. As shown in Figure 2A, the overall survival decreases significantly as the age of diagnosis increases. Figure 2B shows the survival probability of patients of four races except for black patients was > 50% at 80 months. Asian or Pacific Islander patients had the highest survival probability, followed by white and American Indian/Alaska Native patients. Figure 2C shows that once the summary stage starts to progress, the overall survival decreases significantly. AJCC stage IV had the worst survival probability, and the survival probability for AJCC stage 0a-I patients was >50% at 80 months (Figure 2D). Neuroendocrine carcinoma of the bladder has the worst survival probability, while transitional cell carcinoma of the bladder has the best survival probability (Figure 2E). Figure 2F shows that patients who underwent radical cystectomy had the worst survival probability, which was associated with the fact that most of the patients in this group were in an advanced stage of cancer.
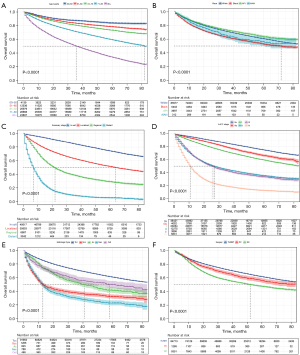
Figure 3 shows the survival probability for patients with five histologic types of BC (transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma, neuroendocrine carcinoma and other epithelial tumors of the bladder) after undergoing different surgical approaches. Patients with transitional cell carcinoma have the highest survival probability after TURBT, in contrast to BC patients with the other four histologic types. Patients with squamous cell carcinoma have the highest survival probability after partial cystectomy, which is similar to adenocarcinoma.
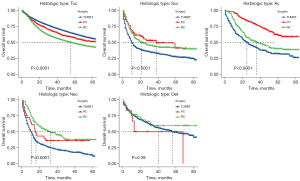
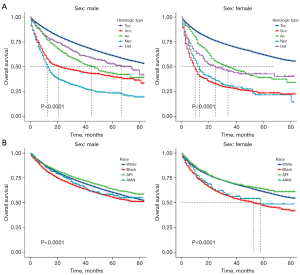
Figure 4A shows that neuroendocrine carcinoma of the bladder had the worst survival probability among male patients, but among female patients, both neuroendocrine carcinoma of the bladder and squamous cell carcinoma of the bladder had a lower survival probability. Figure 4B shows significant differences in survival probability among the four ethnic groups of BC patients, and this was more remarkable in female patients.
Prognostic nomogram
A nomogram was constructed based on the Cox proportional hazard model to predict the 1-, 3-, and 5-year OS in BC patients (Figure 5). Each subgroup variable was assigned a corresponding score to construct this nomogram. A scoring system was used to assign a score from 0 to 100 to each subgroup variable based on its contribution. These scores are added to the registered variables to generate a total score for the bottom scale, which was then converted to predict the corresponding OS.
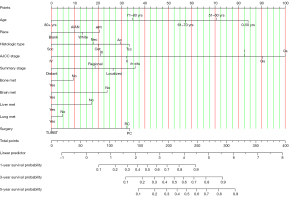
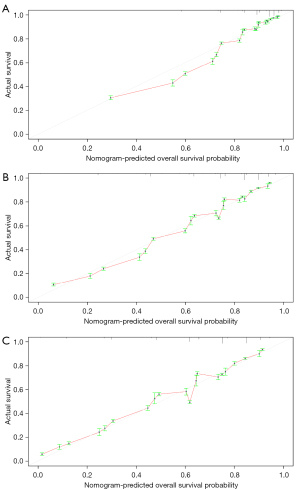
Validation of the nomogram
The calibration curve was used to validate the model’s ability to predict 1-, 3-, and 5-year OS in BC patients. As shown in Figure 6, a perfect correlation between nomogram prediction and observed outcomes demonstrated the great reliability of our nomogram. The C-index is 0.768, which is greater than 0.7. These results suggested that the newly established nomogram was considerably accurate.
Discussion
BC is the ninth most common cancer in the world and one of the most common cancers of the urinary system (2,11). Although our results showed that the overall incidence of BC showed a downward trend, there was still an increasing trend in patients aged >80 years. Despite the fact that we now have more treatment options available for BC patients, including molecular targeted drugs, neoadjuvant chemotherapy, and immunotherapy, older patients do not seem to benefit from these promising therapies.
In this study, we obtained data from 95,329 BC patients, and by the Kaplan-Meier method, we found that the OS of BC patients not only had racial differences but was also related to TNM stage, histological type, and surgery. Meanwhile, a subgroup analysis was performed and showed that OS differed by histological type in patients of different sexes or after different surgical procedures and that OS also differed between female patients of different races.
We found that the number of men with BC was much higher than the number of women, and most patients were aged over 50 years. Most BCs were transitional cell carcinoma. Several studies have previously been performed to illustrate why BC is more likely to occur in men (2,12-15): one reason is the interaction of estrogen, androgens and the liver, and another reason is related to smoking, which increases the risk of BC because more men smoke than women (13,16). In addition, older patients are susceptible to BC due to degeneration of the body’s immune system, leading to an increase in cancer incidence year by year (17-19). Additionally, the decline in bladder function and the development of benign prostatic hyperplasia in male patients as they aged leads to chronic urinary retention (20), which increases exposure to carcinogens and increases the risk of developing BC.
We used univariate and multivariate risk analyses to evaluate various risk factors that have a significant impact on OS in patients with BC. Multivariate analyses showed that all factors, except sex and TNM-M stage, had a significant effect on overall survival in bladder cancer, which is consistent with published studies (6,13,21).
OS was lowest for patients who underwent radical cystectomy due to the higher tumor stage (T2–4), with or without lymph nodes or distant metastases, with approximately 76% of patients being older than 60 years. In contrast, patients who underwent TURBT had the best OS; although more than 80% of this group of patients were older than 60 years, these patients tended to have an earlier tumor stage. Our findings showed that the risk of death increased with age and tumor stage (TNM stage, AJCC stage). A review of the literature shows that as patients age, immunity and physical function decline, which exacerbates the impact of the disease on patients to some extent (22-24). In addition, as patients age, exposure to carcinogens may increase (24), which may affect patient OS. As tumor grading increases, the chances of tumor cell spreading and metastasis increase, which makes treatment more difficult.
The nomogram is widely used to predict OS in oncology patients (25-28). It has been proven that multivariate prediction models predict cancer outcomes more accurately than the cancer stage system (29,30). Our nomogram incorporates more risk factors, which to some extent increases its personalized prediction, and decisions about follow-up and surveillance of cancer patients are made based on risk. Patients predicted to be at higher risk need to receive more intensive follow-up and more accurate treatments, and the inclusion of more risk factors allows us to make more reliable predictions. The C-index of the nomogram was 0.768, indicating that the nomogram is an accurate model for predicting OS.
There are certain shortcomings in this study. First, the SEER database includes less than 30% of the U.S. population, indicating that the scope of the database is not large enough. For example, due to the limited number of patients with TNM-T0 stage, we lacked additional data to analyses the survival differences in patients with T0 stage. Therefore, our survival analyze for this period did not provide additional results. Second, we did not include other factors that may affect OS, such as economic conditions (31), marital status (32), genetic factors (33-35), posttreatment care status (36,37), and patient physical status. The inclusion of these factors would further make the nomogram more accurate. Finally, chemotherapy, radiotherapy and other novel therapy strategies may affect the OS of BC patients. However, some of the SEER data do not provide this important information. Therefore, more studies are needed to identify risk factors that affect OS in BC patients.
Conclusions
Our study showed that the overall incidence of BC has been decreasing year by year since 1992. Meanwhile, we found that age, race, histologic type, tumor stage, and organ metastasis were independent predictors of OS in BC patients. To better predict the 1-, 3-, and 5-year OS in BC patients, we constructed a nomogram based on specific characteristics. The C-index was satisfactory in internal validation. This prediction tool will help physicians assess individualized survival predictions and could provide better surveillance strategies for BC patients.
Acknowledgments
The authors would like to thank all working group members for their contribution to this study.
Funding: This research was funded by the Kuanren Talents Program of Chongqing Medical University (No. KY2019Y026) and the National Natural Science Foundation of China (No. 81803057).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-46/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-46/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of Bladder Cancer. Med Sci (Basel) 2020;8:15. [Crossref] [PubMed]
- Mancini M, Righetto M, Baggio G. Spotlight on gender-specific disparities in bladder cancer. Urologia 2020;87:103-14. [Crossref] [PubMed]
- Brenner DR, Ruan Y, Shaw E, et al. Age-standardized cancer-incidence trends in Canada, 1971-2015. CMAJ 2019;191:E1262-73. [Crossref] [PubMed]
- Trinh QD, Hong F, Halpenny B, et al. Racial/ethnicity differences in endorsing influential factors for prostate cancer treatment choice: An analysis of data from the personal patient profile-prostate (P3P) I and II trials. Urol Oncol 2020;38:78.e7-13. [Crossref] [PubMed]
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol 2016;69:300-10. [Crossref] [PubMed]
- Fang W, Yang ZY, Chen TY, et al. Ethnicity and survival in bladder cancer: a population-based study based on the SEER database. J Transl Med 2020;18:145. [Crossref] [PubMed]
- Dong YM, Sun J, Li YX, et al. Development and Validation of a Nomogram for Assessing Survival in Patients With COVID-19 Pneumonia. Clin Infect Dis 2021;72:652-60. [Crossref] [PubMed]
- Hou C, Cai H, Zhu Y, et al. Development and Validation of Autophagy-Related Gene Signature and Nomogram for Predicting Survival in Oral Squamous Cell Carcinoma. Front Oncol 2020;10:558596. [Crossref] [PubMed]
- Gittleman H, Sloan AE, Barnholtz-Sloan JS. An independently validated survival nomogram for lower-grade glioma. Neuro Oncol 2020;22:665-74. [Crossref] [PubMed]
- Lenis AT, Lec PM, Chamie K, et al. Bladder Cancer: A Review. JAMA 2020;324:1980-91. [Crossref] [PubMed]
- Coleman NC, Burnett RT, Higbee JD, et al. Cancer mortality risk, fine particulate air pollution, and smoking in a large, representative cohort of US adults. Cancer Causes Control 2020;31:767-76. [Crossref] [PubMed]
- Janisch F, Shariat SF, Schernhammer E, et al. The interaction of gender and smoking on bladder cancer risks. Curr Opin Urol 2019;29:249-55. [Crossref] [PubMed]
- Liss MA, White M, Natarajan L, et al. Exercise Decreases and Smoking Increases Bladder Cancer Mortality. Clin Genitourin Cancer 2017;15:391-5. [Crossref] [PubMed]
- Mori K, Mostafaei H, Abufaraj M, et al. Smoking and bladder cancer: review of the recent literature. Curr Opin Urol 2020;30:720-5. [Crossref] [PubMed]
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Osterman CK, Deal AM, McCloskey H, et al. Impairment and Longitudinal Recovery of Older Adults Treated with Radical Cystectomy for Muscle Invasive Bladder Cancer. J Urol 2021;205:94-9. [Crossref] [PubMed]
- Garg T, Young AJ, O'Keeffe-Rosetti M, et al. Association between metabolic syndrome and recurrence of nonmuscle-invasive bladder cancer in older adults. Urol Oncol 2020;38:737.e17-23. [Crossref] [PubMed]
- Mottet N, Ribal MJ, Boyle H, et al. Management of bladder cancer in older patients: Position paper of a SIOG Task Force. J Geriatr Oncol 2020;11:1043-53. [Crossref] [PubMed]
- Aluwini S, van Rooij PH, Kirkels WJ, et al. Bladder function preservation with brachytherapy, external beam radiation therapy, and limited surgery in bladder cancer patients: Long-term results Int J Radiat Oncol Biol Phys 2014;88:611-7. [corrected]. [Crossref] [PubMed]
- Sadighian M, Porten S. Gender differences in oncologic and functional outcomes in patients with bladder cancer undergoing radical cystectomy with urinary diversion. Curr Opin Urol 2019;29:542-7. [Crossref] [PubMed]
- Rosiello G, Palumbo C, Deuker M, et al. Sex- and age-related differences in the distribution of bladder cancer metastases. Jpn J Clin Oncol 2021;51:976-83. [Crossref] [PubMed]
- Fletcher SA, Cole AP, Lu C, et al. The impact of underinsurance on bladder cancer diagnosis, survival, and care delivery for individuals under the age of 65 years. Cancer 2020;126:496-505. [Crossref] [PubMed]
- Krajewski W, Rodríguez Faba O, Breda A, et al. Analysis of age influence on oncological results and toxicity of BCG immunotherapy in non-muscle invasive bladder cancer. World J Urol 2020;38:3177-82. [Crossref] [PubMed]
- Yang Y, Liang S, Geng J, et al. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care 2020;8:45. [Crossref] [PubMed]
- Dong D, Fang MJ, Tang L, et al. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multicenter study. Ann Oncol 2020;31:912-20. [Crossref] [PubMed]
- Wang S, Tu J. Nomogram to predict multidrug-resistant tuberculosis. Ann Clin Microbiol Antimicrob 2020;19:27. [Crossref] [PubMed]
- Liang W, Yang P, Huang R, et al. A Combined Nomogram Model to Preoperatively Predict Histologic Grade in Pancreatic Neuroendocrine Tumors. Clin Cancer Res 2019;25:584-94. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Kattan MW, et al. Clinical benefits of a multivariate prediction model for bladder cancer: a decision analytic approach. Cancer 2009;115:5460-9. Erratum in: Cancer 2011;117:3867. [Crossref] [PubMed]
- International Bladder Cancer Nomogram Consortium. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967-72. [Crossref] [PubMed]
- Zang Y, Li X, Cheng Y, et al. An overview of patients with urothelial bladder cancer over the past two decades: a Surveillance, Epidemiology, and End Results (SEER) study. Ann Transl Med 2020;8:1587. [Crossref] [PubMed]
- Tao L, Pan X, Zhang L, et al. Marital Status and Prognostic Nomogram for Bladder Cancer With Distant Metastasis: A SEER-Based Study. Front Oncol 2020;10:586458. [Crossref] [PubMed]
- Liu H, Gu J, Jin Y, et al. Genetic variants in N6-methyladenosine are associated with bladder cancer risk in the Chinese population. Arch Toxicol 2021;95:299-309. [Crossref] [PubMed]
- Mojarrad M, Moghbeli M. Genetic and molecular biology of bladder cancer among Iranian patients. Mol Genet Genomic Med 2020;8:e1233. [Crossref] [PubMed]
- Guo Z, Zhu H, Xu W, et al. Alternative splicing related genetic variants contribute to bladder cancer risk. Mol Carcinog 2020;59:923-9. [Crossref] [PubMed]
- Allen BC, Oto A, Akin O, et al. ACR Appropriateness Criteria(R) Post-Treatment Surveillance of Bladder Cancer: 2021 Update. J Am Coll Radiol 2021;18:S126-38. [Crossref] [PubMed]
- Vartolomei L, Vartolomei MD, Shariat SF. Bladder Cancer: Depression, Anxiety, and Suicidality Among the Highest-risk Oncology Patients. Eur Urol Focus 2020;6:1158-61. [Crossref] [PubMed]


