
Ovarian metastases from ALK-positive lung adenocarcinoma: a case report and review of the literature
Introduction
Lung cancer is one of the most common malignant tumors. It is prone to metastasize to the brain, bone, liver, and adrenal glands, but rarely to the ovary. It has been reported that only around 5% of lung cancer patients experienced ovarian metastasis (1,2). Few lung adenocarcinoma patients with ovarian metastases were diagnosed from the metastasis, and it is extremely difficult to distinguish from primary ovarian cancer. Here we report a case with anaplastic lymphoma kinase (ALK)-positive primary lung cancer who experienced ovarian metastasis at the age of only 23-year-old. Compared with the previous reports of lung carcinoma with ovarian metastases, we provided the youngest case. The patient had no symptoms related to primary lung adenocarcinoma, but an abdominal mass caused by ovarian metastasis was discovered by accidence. Postoperative pathology and immunohistochemical analysis proved that the patient’s ovarian tumor originated from the lung. We also explored the clinical features, diagnosis, and treatments of ALK-positive lung carcinoma patients with ovarian metastases, and reviewed the relevant kinds of literature. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-273/rc) (3).
Case presentation
A 23-year-old woman with no prior smoking history came to a local clinic for treatment in February 2021 due to a lump in her abdomen when she was lying supine, without significant abdominal pain or weight change. On March 19, 2021, the patient consciously came to our hospital because of frequent urination for nearly half a month. Physical examination in the clinic revealed that a cystic mass could be touched on the left side of the uterus, with clear borders, poor mobility, and no tenderness. The gynecological ultrasound images showed a mass (13.13 cm × 9.71 cm × 11.68 cm) was located in the anterior upper part of the uterus with the clear boundary and the inner anechoic chamber.
After admission, further pre-operative inspections were done. Serum tumor markers on March 19, 2021, such as carcinoembryonic antigen (CEA; 54.99 ng/mL), carbohydrate antigen 125 (CA125; 422.1 U/mL), and carbohydrate antigen 153 (CA153; 72.05 U/mL) were significant increase. Whole abdominal computed tomography (CT) showed a huge mass (mainly cystic but with solid partitions inside) occupying the front of the uterus, and its size was about 10.2 cm × 10.3 cm × 14.1 cm (Figure 1A). Unexpectedly, a chest CT examination revealed an irregular solid nodule of 2.0 cm × 1.3 cm in the middle/outer segment of the right lung (Figure 1A). After fully informed consent, on March 22, 2021, the patient underwent laparoscopy followed by an uncomplicated bilateral ovarian tumor resection to confirm the nature of the tumor. Specifically, the right ovary exhibited a multilocular cystic-solid mass of 14 cm × 12 cm, adhering to the lateral pelvic wall. Meanwhile, the left ovary showed a bit of unanticipated enlargement, and a 3 cm × 2 cm tumor was found after ovarian dissection. The uterus, bilateral fallopian tubes, omentum and appendix appeared in regular size and no morphological changes were found. However, the intraoperative frozen section biopsy provided very limited information and was only diagnosed as bilateral ovaries malignancy but unknown origin. Considering the pathological uncertainty and the young age of the patient, the following comprehensive surgical staging was not performed at that point.
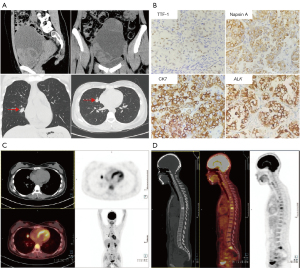
The later convention pathology examination confirmed the findings in the frozen section biopsy and diagnosed the tumor as moderately-poorly differentiated serous adenocarcinoma, which needed examination to exclude metastatic carcinoma. However, no abnormal findings were found in gastrointestinal endoscopy and colonoscopy, and the origin of the ovarian tumor remained unclear. The results of subsequent IHC detection were shown as follows: thyroid transcription factor-1 (TTF-1) (+), Napsin A (+), cytokeratin 7 (CK7) (+), ALK (Ventana-D5F3) (+), cytokeratin 20 (CK20) (−), Paired Box 8 (PAX8) (−), P53 (−) and tumor proportion score (TPS) of programmed death-ligand 1 (PD-L1) 22C3 (Dako +) = around 15% (Figure 1B). Taking these together, the patient was diagnosed with primary lung cancer with ovarian metastasis. On April 5, 2021, the patient underwent an intravenous injection of 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG) in a fasting state and received whole-body positron emission tomography combined with low-dose computed tomography (PET/CT) tomography (Figure 1C,1D). The report was described as follows: (I) irregular nodules with varying sizes were found in the middle lobe of the right lung, and the largest one was about 17 mm × 14 mm. The boundary of some lesions was not clear, but fluorodeoxyglucose (FDG) uptake was increased with standard uptake value (SUV)max =9.4. (II) Multiple lymph node metastases were found in retroperitoneum, right cardia, subcarinal space, right lung hilum, posterior-anterior vena cava, para-aortic arch, both sides of the thoracic entrance vascular space, both sides of the supraclavicular fossa, and left posterior cervical triangle. The short diameter of the largest lymph node was approximately 8 mm and FDG intake was also increased with SUVmax =5.0. (III) Multiple bone metastases were observed in the vertebrae, both sides of the acetabulum, and left ischia with SUVmax =4.9. In addition, the hybrid capture method combined with second-generation high-throughput sequencing technology was used to detect pathogenic/probable pathogenic variants in the DNA with paraffin tissue and the results were as follows: (I) EML4_ALK (E14: A20) gene fusion, mutation abundance 14.61%; (II) TP53 (NM_000546.5) exon 7 frameshift mutation c.770_772delinsC (p. Leu257fs), AF 34. 99%.
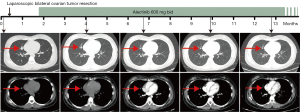
In summary, the patient was finally diagnosed with moderately-poorly differentiated serous lung adenocarcinoma with ovarian metastasis, and the clinical stage was T1N3M1 (stage IV). Based on genetic test results, alectinib administration was started at doses of 600 mg twice daily (bid) on April 22, 2021. Initially, no significant adverse events but common side effects such as headache, dizziness and myalgia were developed. After symptomatic treatment, the patient’s condition was stable. During follow-up, the patient received a quarterly CT scan approximately, showing an acceptable clinical response up to the present (Figure 2). Specifically, the CT scan on July 8, 2021 (the 4th month in Figure 2) showed a relatively smaller solid nodule (about 1.02 cm × 1.96 cm) in the middle lobe of the right lung with burrs on the edge. However, little change in the mass was further observed on September 23 (the 6th–7th month in Figure 2). On January 5, 2022 (the 9th–10th month in Figure 2), the patient’s CT scan exhibited another significant remission for the lobe mass (about 0.84 cm × 0.96 cm). The latest CT scan on March 29 showed the mass to be 0.77 cm × 0.94 cm (the 12th–13th month in Figure 2).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Lung cancer is the most common malignant tumor and the main cause of human cancer deaths worldwide (4,5) with a 5-year survival rate of 10–20% (6). Furthermore, a study has shown that the median survival after diagnosis was 13 months for non-metastatic lung cancer and only 5 months for metastatic lung cancer (7). Ovarian metastases are not common, which account for approximately 10–30% of all ovarian cancers (8). Most of the metastases come from the female reproductive system, such as the contralateral ovary and uterus (9). Only 2–4% of ovarian metastatic masses come from the lung (1). The 5-year survival rate of patients with metastatic ovarian cancer is lower than those with primary ovarian cancer (10). However, the frequency of ovarian metastasis is increasing with the rising incidence of lung cancer in women (11,12).
In addition, an increasing trend of lung cancer patients with ovarian metastases among young patients, which may be attributed to the vigorous ovarian function and abundant lymphatic and blood supply in the young patients (11). Then the special structure of the ovary has a certain relationship with the occurrence of metastasis. The ovaries are located in the lower part of the pelvic and abdominal cavity, where are prone to be planted and metastasized. In addition, the defects and epithelial depressions on the ovarian surface formed during ovulation may also be beneficial to the cultivation of cancer cells (13).
The therapeutic strategy for primary ovarian cancer is to maximize the removal of the tumor to bring better survival benefits to the patient (14). However, the prognosis of patients with ovarian metastasis is dismal and the resection of ovarian metastases has little effect on the prognosis. Due to differences in survival rates and treatment methods, distinguishing between primary and secondary ovarian malignancies is meaningful for deciding treatment strategies for the patients (9).
In clinical practice, in order to identify whether the ovarian tumor is primary or secondary, patients are generally subjected to various tests, including ultrasonography, CT, magnetic resonance imaging (MRI), and tests of tumor markers. Solid tumor masses with clear borders and smooth cyst walls are more frequently found for secondary ovarian malignancies by ultrasonography (15). Unclear boundaries, multiple dark areas, thick and uneven walls, as well as solid inside echoes are usually found in ultrasonography for primary ovarian tumors. CT and MRI of metastatic ovarian tumors usually show bilateral, lobulated, clear-bounded oval cystic solid masses, and the outlines of the ovaries are basically maintained (16), which are similar to the findings in the present case.
The biopsy is the most accurate preoperative method to identify primary and metastatic tumors and laparoscopic ovarian tumor biopsy is useful and safe for histological diagnosis (17). With the further application of Sanger sequencing or amplification refractory mutation system (ARMS) and fluorescence in situ hybridization (FISH) in the clinic, the source of ovarian metastases can be determined by detecting the pathological and immunohistochemical characteristics of the tumor (18).
Ovarian metastases of lung cancer are reportedly rare. Previously reports of lung cancer with ovarian metastases reported various pathological types, including adenocarcinoma, small cell carcinoma, large cell carcinoma, and squamous cell carcinoma, however, the youngest case was aged 25 years (19), a bit older than the patient in this report. Furthermore, more than a half of the previously reported cases were firstly found to have lung tumors but not ovarian tumors; one-third of the cases were simultaneously found to have both tumors; and the remaining cases were firstly found to have ovarian tumors and then diagnosed as primary lung cancer (11,20), which is similar to our case. At present, the case reported here is the youngest ALK-positive lung cancer patient with ovarian metastasis, and the case had no cough, hemoptysis, and other related symptoms caused by lung cancer.
The IHC features of ovarian tumors allowed us to confirm ovarian tumors originated from lung cancer. Moreover, we also searched the literature and obtained 12 reported cases with specific molecular changes or ALK-positive (Table 1). Our case and the 12 cases were aged from 23 to 72 years with an average of 44.5 years. Among the 13 patients, one was a mild smoker (2.5 pack-years), another one smoked one pack per day, and the remaining patients had no smoking history. Totally, 7 patients were ALK-positive, and 10 patients had lung cancer-related immunohistochemical findings.
Table 1
| Age (years) | Smoking history | Clinical history | Histopathology | Surgical procedure | Other metastases | ALK | Immuno-histochemistry | References |
|---|---|---|---|---|---|---|---|---|
| 47 | No | Cough | Lung adenocarcinoma | BSO | Bone brain breast | Positive | Napsin A+, CK7+, TTF-1+ | (21) |
| 25 | No | Cough | SCLC | Left adnexectomy | Vertebral | – | CK7+, TTF-1+ | (19) |
| 60 | – | Cough and dyspnea | Lung adenocarcinoma | Tumor debulking surgery | Peritoneum, bladder, uterine, intestinal tubes | – | TTF-1+, CK7+, Napsin A+ | (22) |
| 43 | No | Cough, dyspnea and chest pain | Lung adenocarcinoma | TAH-BSO | Pleura | – | CK7+, CK20−, TTF-1+ | (23) |
| 41 | No | Cough and dyspnea | NSCLC | Torsion resection of ovarian tumor | – | Positive | TTF-1+, Napsin A+ | (24) |
| 37 | No | Headache, nausea, and appetite loss | Lung adenocarcinoma | – | Brain | Positive | – | (25) |
| 63 | No | Cough and hemoptysis | Lung adenocarcinoma | BSO | Pleura | – | CK7+, CK20−, TTF-1+ | (26) |
| 33 | No | Pulmonary mass and adnexal mass | Lung adenocarcinoma | – | – | Positive | CK7+, TTF-1+, Napsin A+ | (27) |
| 54 | 2.5 pack-years | Cough and dyspnea | NSCLC | BSO | Brain | Positive | – | (28) |
| 72 | No | Vaginal bleeding | SCLC | TAH-BSO | – | – | TTF-1+, c-Kit+, EMA+, CK20− | (20) |
| 42 | No | – | SCLC | Salpingo-oophorectomy | – | – | TTF-1+, synaptophysin+ | (29) |
| 39 | 1 pack-day | Persistent cough | NSCLC | Left salpingo-oophorectomy and right ovarian cystectomy | Brain | Positive | – | (30) |
| 23 | No | Abdominal lumps | Lung adenocarcinoma | BSO | Bone | Positive | CK7+, CK20−, TTF-1+ | – |
ALK, anaplastic lymphoma kinase; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; BSO, bilateral salpingo-oophorectomy; TAH, total abdominal hysterectomy; CK7, cytokeratin 7; TTF-1, thyroid transcription factor-1; CK20, cytokeratin 20.
TTF-1 can be used as a highly sensitive and specific immunomarker to differentiate metastatic pulmonary from extrapulmonary adenocarcinoma (31,32). TTF-1 is a homeodomain transcription factor required for lung morphogenesis and epithelial cell differentiation (33). It can be detected in thyroid follicular cells, neonatal alveolar cell clocks, and in lung adenocarcinoma and poorly differentiated lung squamous cell. Napsin A is an aspartic protease, which is mainly found in type II pneumocytes and lung macrophages (34). Napsin A is highly expressed in adenocarcinomas of the lung and is commonly used as an immunohistochemical index to identify lung cancer metastases. Therefore, the combined detection of Napsin A and TTF-1 can be used to identify the second tumors originating from lung adenocarcinoma or other sites, and have improved sensitivity and specificity in identifying metastatic adenocarcinomas of lung origin.
CK7 and CK20 are both low-molecular-weight cytokeratin compounds, which are expressed in epithelial cells and can be used to identify the primary site of metastatic adenocarcinoma originating from colorectal, ovary or lung (35-37). More than 90% of colon-derived adenocarcinomas are CK7 (−) and CK20 (+), and over 90% of ovarian-derived adenocarcinomas and more than 80% of endometrial, breast, and lung-derived adenocarcinomas are CK7 (+) and CK20 (−) (38). In the present case, immunohistochemical staining results were CK7 (+), CK20 (−) and TTF-1 (+), which is in line with the diagnosis of lung adenocarcinoma with ovarian metastasis. In summary, the imaging features and immunohistochemical staining are very useful for distinguishing between primary and metastatic ovarian tumors.
A relationship between driver gene mutations and distant metastases in lung adenocarcinoma has been frequently reported, and the diagnostic value of the driver gene mutation status in optimizing treatment regimens is now well-accepted. Lung adenocarcinoma with ALK gene rearrangement is a specific molecular subtype of lung adenocarcinoma discovered in recent years. IHC can detect ALK fusion proteins positive in lung cancer (39). It is common in the young and non-smokers with lung adenocarcinoma, accounting for about 3% to 5% of non-small cell lung cancer (NSCLC) (40).
ALK rearrangement is a common molecular alteration in lung tumors with ovarian metastases. Alectinib is a common treatment option for ALK-positive lung adenocarcinoma patients with ovarian metastasis. Alectinib is a new type of highly targeted second-generation ALK-tyrosine kinase inhibitor (ALK-TKI). Clinical studies have shown that it has high blood-brain barrier permeability and central nervous system (CNS) permeability, and its inhibitory effect on ALK-positive tumor cells is 5 times stronger than that of Crizotinib (41). In the global phase III (ALEX) study, when alectinib was used in the first-line treatment of patients with advanced ALK-NSCLC, it showed excellent efficacy, with progression-free survival (PFS) as high as 34.8 months (42). In 2018, the National Comprehensive Cancer Network (NCCN) recommended alectinib as the first-line treatment for ALK-NSCLC patients (43). In this case, the patient is taking alectinib 600 mg bid continuously and has achieved a significant clinical response up to the present.
To sum up, ovarian metastases from lung cancer are relatively rare in clinical practice, and whether ALK-positive patients are more likely to develop ovarian metastases still needs further observation. Based on our experiences (combined with the postoperative IHC, imaging reports, and genetic testing results), a rare metastatic ovarian tumor with primary lung cancer disease can still be diagnosed. Considering the frequency of ovarian metastases is increasing due to the rising incidence of lung cancer in women, clinicians should consider the possibility of lung cancer metastases when ovarian occupation occurs. In addition, our study provided evidence that alectinib can be considered a treatment option for these ALK-positive patients. Limitations of our study include the inadequate follow-up period and a lack of beneficial comparison with the patients who did not receive alectinib. Only one case in the present study also limited its credibility.
Conclusions
Ovarian metastases originating from lung adenocarcinoma are extremely rare. We report a young (23-year-old) patient with ovarian metastasis originated from ALK-positive lung adenocarcinoma. The patient had no symptoms related to primary lung adenocarcinoma, and the first symptom was an abdominal mass caused by ovarian metastasis. In addition, identifying the source of ovarian tumors is critical to deciding patient treatment strategies, which can be helped by IHC tests. The clinical use of alectinib provides a good treatment option for ALK-positive patients. However, whether or not the IHC tests together with alectinib treatment can replace invasive biopsy in ALK-positive patients to determine the origin of ovarian tumors, as well as whether or not alectinib combined with local surgery or radiation may bring sustained survival benefits deserve further study.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-273/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-273/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jung YE, Lee JW, Kim BG, et al. Ovarian metastasis from pulmonary adenocarcinoma. Obstet Gynecol Sci 2013;56:341-4. [Crossref] [PubMed]
- Testa AC, De Blasis I, Di Legge A, et al. Ovarian metastasis from adenocarcinoma of the lung. Ultrasound Obstet Gynecol 2013;42:241-2. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Alvarado-Cabrero I, Rodríguez-Gómez A, Castelan-Pedraza J, et al. Metastatic ovarian tumors: a clinicopathologic study of 150 cases. Anal Quant Cytopathol Histpathol 2013;35:241-8. [PubMed]
- Li W, Wang H, Wang J, et al. Ovarian metastases resection from extragenital primary sites: outcome and prognostic factor analysis of 147 patients. BMC Cancer 2012;12:278. [Crossref] [PubMed]
- Skírnisdóttir I, Garmo H, Holmberg L. Non-genital tract metastases to the ovaries presented as ovarian tumors in Sweden 1990-2003: occurrence, origin and survival compared to ovarian cancer. Gynecol Oncol 2007;105:166-71. [Crossref] [PubMed]
- Irving JA, Young RH. Lung carcinoma metastatic to the ovary: a clinicopathologic study of 32 cases emphasizing their morphologic spectrum and problems in differential diagnosis. Am J Surg Pathol 2005;29:997-1006. [Crossref] [PubMed]
- Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005;128:370-81. [Crossref] [PubMed]
- Hauptmann S. Differential diagnosis of ovarian metastases. Pathologe 2007;28:215-21. [Crossref] [PubMed]
- Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol 2007;25:2873-83. [Crossref] [PubMed]
- Antila R, Jalkanen J, Heikinheimo O. Comparison of secondary and primary ovarian malignancies reveals differences in their pre- and perioperative characteristics. Gynecol Oncol 2006;101:97-101. [Crossref] [PubMed]
- Brown DL, Zou KH, Tempany CM, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology 2001;219:213-8. [Crossref] [PubMed]
- Odajima S, Ueda K, Hosoya S, et al. Clinical Availability of Tumour Biopsy Using Diagnostic Laparoscopy for Advanced Ovarian Cancer. In Vivo 2021;35:3325-31. [Crossref] [PubMed]
- Young RH, Scully RE. Ovarian metastases from cancer of the lung: problems in interpretation--a report of seven cases. Gynecol Oncol 1985;21:337-50. [Crossref] [PubMed]
- Varlas VN, Angelescu G, Rhazi Y, et al. Challenges of an ovarian neuroendocrine metastasis of advanced small-cell lung carcinoma - literature review and case report. Acta Endocrinol (Buchar) 2021;17:251-8. [Crossref] [PubMed]
- Oneda E, Zorzi F, Gorio A, et al. Differential Diagnosis of Small Cell Carcinoma of the Ovary or Ovarian Metastases of Small Cell Carcinoma of the Lung: A Case Report and Review of the Literature. Case Rep Oncol 2020;13:822-8. [Crossref] [PubMed]
- Mushi RT, Yang Y, Cai Q, et al. Ovarian metastasis from non-small cell lung cancer with ALK and EGFR mutations: A report of two cases. Oncol Lett 2016;12:4361-6. [Crossref] [PubMed]
- Yao S, Wang L, Tian X, et al. Lung Adenocarcinoma with Metachronous Ovarian Metastasis: a long survival case report. BMC Womens Health 2021;21:152. [Crossref] [PubMed]
- Franchina T, Russo A, Ricciardi GR, et al. Long time response with chemotherapy in ROS1 NSCLC patient with unusual metastatic site. Cancer Biol Ther 2016;17:1089-93. [Crossref] [PubMed]
- Jing X, Li F, Meng X, et al. Ovarian metastasis from lung adenocarcinoma with ALK-positive rearrangement detected by next generation sequencing: A case report and literatures review. Cancer Biol Ther 2017;18:279-84. [Crossref] [PubMed]
- Sasano H, Sekine A, Hirata T, et al. Ovarian Metastases from ALK-rearranged Lung Adenocarcinoma: A Case Report and Literature Review. Intern Med 2018;57:3271-5. [Crossref] [PubMed]
- Yeh KY, Chang JW, Hsueh S, et al. Ovarian metastasis originating from bronchioloalveolar carcinoma: a rare presentation of lung cancer. Jpn J Clin Oncol 2003;33:404-7. [Crossref] [PubMed]
- Wang W, Wu W, Zhang Y. Response to crizotinib in a lung adenocarcinoma patient harboring EML4-ALK translocation with adnexal metastasis: A Case Report. Medicine (Baltimore) 2016;95:e4221. [Crossref] [PubMed]
- Lee KA, Lee JS, Min JK, et al. Bilateral Ovarian Metastases from ALK Rearranged Non-Small Cell Lung Cancer. Tuberc Respir Dis (Seoul) 2014;77:258-61. [Crossref] [PubMed]
- Kitazawa J, Takahashi A, Uemura M, et al. Small-cell lung carcinoma with ovarian metastasis 4 years after the first-line treatment. Int Cancer Conf J 2019;8:109-13. [Crossref] [PubMed]
- Fujiwara A, Higashiyama M, Kanou T, et al. Bilateral ovarian metastasis of non-small cell lung cancer with ALK rearrangement. Lung Cancer 2014;83:302-4. [Crossref] [PubMed]
- Di Loreto C, Di Lauro V, Puglisi F, et al. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol 1997;50:30-2. [Crossref] [PubMed]
- Ng WK, Chow JC, Ng PK. Thyroid transcription factor-1 is highly sensitive and specific in differentiating metastatic pulmonary from extrapulmonary adenocarcinoma in effusion fluid cytology specimens. Cancer 2002;96:43-8. [Crossref] [PubMed]
- Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev 2007;87:219-44. [Crossref] [PubMed]
- Weidemann S, Böhle JL, Contreras H, et al. Napsin A Expression in Human Tumors and Normal Tissues. Pathol Oncol Res 2021;27:613099. [Crossref] [PubMed]
- Berezowski K, Stastny JF, Kornstein MJ. Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol 1996;9:426-9. [PubMed]
- Wauters CC, Smedts F, Gerrits LG, et al. Keratins 7 and 20 as diagnostic markers of carcinomas metastatic to the ovary. Hum Pathol 1995;26:852-5. [Crossref] [PubMed]
- Loy TS, Calaluce RD. Utility of cytokeratin immunostaining in separating pulmonary adenocarcinomas from colonic adenocarcinomas. Am J Clin Pathol 1994;102:764-7. [Crossref] [PubMed]
- Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 2000;13:962-72. [Crossref] [PubMed]
- Bi R, Bai Q, Zhu X, et al. ALK rearrangement: a high-frequency alteration in ovarian metastasis from lung adenocarcinoma. Diagn Pathol 2019;14:96. [Crossref] [PubMed]
- Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol 2009;4:1450-4. [Crossref] [PubMed]
- Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol 2018;36:1199-206. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]


