
Perioperative management of intrahepatic cholangiocarcinoma patients with hereditary coagulation factor V deficiency: a case report and literature review
Introduction
Coagulation factor V (FV) is a coagulation-related glycoprotein with high molecular weight (330 KD). It is an in vivo protein cofactor required for the rapid production of thrombin catalyzed by prothrombin complex, which can increase the activation rate of thrombin by more than 1,000 times. Plasma FV has dual effects of promoting coagulation and anticoagulation, and plays a key role in hemostasis and thrombosis (1). Concomitant FV deficiency is a rare autosomal recessive disorder with an incidence of approximately one in one million that results in a prolongation of prothrombin time (PT) and clinical bleeding (2). Therefore, surgical treatment for patients with coagulation FV deficiency has higher bleeding risk and mortality, and major hepatectomy for such patients has not been reported before. Herein, we presented a case of intrahepatic cholangiocarcinoma (IHC) complicated with FV deficiency who underwent laparoscopic left hemihepatectomy successfully. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-528/rc).
Case description
This case involved a 54-year-old Chinese female with a height of 160 cm and a weight of 52 kg, who was admitted to our hospital because of liver tumor by examination on November 15, 2020 (Figure 1). There was no abdominal pain, nausea, vomiting, fever and other discomfort at admission, and no obvious positive signs of heart, lung and abdomen were found during physical examination. The patient was found abnormal blood coagulation function in an external hospital for more than 1 year. Once hemophilia was suspected without further diagnosis and treatment. No obvious abnormality was found in blood routine and biochemical examination after admission. Admission laboratory tests confirmed an obviously prolonged international normalized ratio INR: 2.05 (0.79–1.12), PT: 22.4 s (9.8–12.9 s), activated partial thromboplastin time (APTT): 39.6 s (23.3–32.5 s). The quantification of alpha fetoprotein was 10.60 ng/mL, and the ratio of AFP-L3/AFP was 76.50%. The detection values of PIVKA II and CA199 were 14.00 mAU/mL and 1.7 U/mL respectively. The coagulation FV activity was 2.6% (62–139%), indicating that the FV activity is significantly decreased. Liver MRI revealed that: (I) malignant tumors of the right anterior lobe and left inner lobe above the gallbladder fossa, with a range of about 9.9*6.4*4.8 cm, were considered to be combined hepatocellular-cholangiocarcinoma (CHC) with hilar and retroperitoneal lymph node metastasis. (II) Chronic cholecystitis, cholecystolithiasis. (III) Small cyst of liver. The final diagnoses were: (I) liver tumor, possibly CHC; (II) cholecystolithiasis with cholecystitis; (III) hepatic cyst; (IV) hepatitis B virus carrier; (V) hemophilia: FV deficiency; (VI) liver cirrhosis. In view of the patient's liver malignant tumor and indications for surgery, an operation with complete surgical resection was the recommended treatment. However, considering the high risk of surgical bleeding caused by hemophilia, the family members chose conservative drug treatment. Then, hepatic transcatheter arterial chemoembolization (TACE) was performed on November 27, 2020, and drug-loaded microspheres (Epirubicin hydrochloride 60 mg) were infused. The treatment of Camrelizumab (200 mg, q3W) was performed on December 2, December 30, 2020 and January 21, 2021 respectively without any side effects of immunotherapy. The patient began to take Anlotinib Hydrochloride (12 mg, D1-D14, Q3W) on December 5, 2020 with no side effects of targeted treatment. After two months of treatment, it was found that the tumor was obviously necrotic, and the hilar area and retroperitoneal lymph nodes were significantly reduced (Figure 1B-1D) on January 19, 2021. Considering the effectiveness of drug treatment, the family members are advised to carry out surgical treatment, but they still refused the operation and insisted on immune combined targeted therapy. The re-examination of liver MRI showed that the area of the tumor was enlarged and there were multiple nodules adjacent to the tumor on April 1, 2021, which were considered as recurrence of liver cancer or intrahepatic metastasis (Figure 1E). We suggested surgical treatment again, and this time the family agreed to accept surgical treatment and actively complete preoperative examination and preparation.
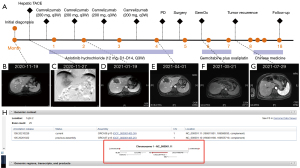
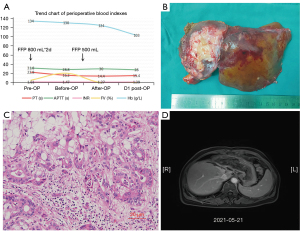
To further confirm the diagnosis of FV deficiency disease, the genetic testing revealed a rare homozygous mutation of the FV gene (rs747215273: FV, c.286G>C, p.D96H, Figure 1H). Therefore, the patient was diagnosed with hereditary coagulation FV deficiency. Preoperative hemagglutination tests showed PT: 22.8 s, INR: 2.17, APTT: 42.7 s, D-dimer: 0.08 mg/L. The coagulation FV activity was 5.4% (62–139%). Two days before the operation, 800 mL (about 15 mL/kg) of fresh frozen plasma (FFP) was injected daily. A review of coagulation function in the morning of the operation day indicated that the coagulation FV activity was 20.6%. The blood coagulation indicated PT: 16.2 s, INR: 1.47, APTT: 28.8 s, D-dimer: 0.10 mg/L (Figure 2A). The laparoscopic left hemihepatectomy (Figure 2B) and cholecystectomy were performed on April 12, 2021, and the operation was smooth with a total time of 170 minutes, 300 mL of intraoperative bleeding and 500 mL of FFP infusion. After the surgery, the patient was extubated and admitted to our intensive care unit. There were no complications such as abdominal bleeding, bile leakage and so on until discharge during the postoperative hospitalization for 10 days. The specimen pathology (Figure 2C) suggested that: (I) gallbladder: chronic cholecystitis, cholelithiasis. (II) Left half liver: primary massive cholangiocarcinoma of the liver, with massive necrosis, interstitial fibrous tissue hyperplasia, invading the liver capsule. MVI grade: M1. Immunohistochemistry staining showed Ki67+, CK7+++, CK18++, CK19++, CD34+, CD10+, Glypican3−, Heppar1−, GS++, Arg−. Instead, the final diagnosis was IHC (pT3NxM0, IIIA). One month after surgery, there was no abnormality in tumor indexes of AFP and CA199, and no tumor recurrence was found in contrast-enhanced MRI scan (Figure 1F,2D) and the patient resumed “Camrelizumab + Anlotinib Hydrochloride” treatment and began to add GemOx (Gemcitabine 1,500 mg ivgtt d1, d8 plus Oxaliplatin 120 mg ivgtt d2) regimen chemotherapy. Although the tumor recurred 3 months after the operation (Figure 1G), the patient was only treated with oral traditional Chinese medicine because she couldn’t tolerate chemotherapy due to adverse reactions such as nausea and vomiting. The patient currently has an overall survival time of 18 months and is still followed-up (Figure 1A).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
FV is distributed 80% in plasma and 20% in platelets, synthesized by liver and megakaryocytes. The FV gene, which is located on the chromosome 1q23, contains 24 introns and 25 exons, which spans the region of about 80 kb and encodes 2,224 amino acid proteins. FV deficiency can be divided into hereditary FV deficiency and acquired FV deficiency. Acquired FV deficiency is associated with drugs, malignant tumors, autoimmune diseases, pregnancy and infection. The patient had liver malignant tumor, so we should pay attention to exclude the possibility of acquired FV deficiency. Therefore, we further performed gene testing to confirm that it is hereditary FV deficiency. Currently, there are about 180 kinds of F5 mutations related to FV deficiency, including synonymous mutations, missense mutations, nonsense mutations, splice site mutations, insertion and deletion, etc. (3). The activity of FV in patients with FV deficiency was significantly decreased, but the activity was not consistent with the degree of clinical bleeding. The clinical bleeding phenotype showed a certain heterogeneity (2). The severity of bleeding in patients with severe FV deficiency was related to the mutation type (4). The European Network of Rare Bleeding Disorders (EN-RBD) and British Committee for Standards in Haematology (BCSH) have established severe disease as undetectable FV levels, moderate disease as factor less than 0.1 U/mL (<10%) and mild disease as FV at least 0.1 U/mL (≥10%) (5). Therefore, this patient was defined as a moderate disease. Due to the lack of concentrated FV, the perioperative management of patients with congenital FV deficiency requires a transfusion protocol with FFP. FFP generally contains approximately 1 IU/mL of FV, and 10-to-20 mL/kg of FFP is recommended to achieve a target safe activity level of 15–20% prior to the invasive procedure (6,7). Platelets transfusion might be necessary in severe cases (8). Platelets contain significant stores of FV and may be able to efficiently deliver FV to sites of vascular injury, even in the absence of plasma FV (9). Although the hemorrhagic risk, the final outcome is often favorable as long as early diagnosis and suitable perioperative management (6,7) which is a real challenge for anesthetists, hematologists and surgeons to work in close collaboration. Finally, we checked the activity of FV free of charge to the patient’s immediate family members, and all of them were normal.
The patient was considered to be a CHC before surgery. According to the imaging result, we suggested that patients can undergo extensive hepatectomy to achieve the RO resection, and it should be the most effective treatment at present (10). However, the family members decided to receive conservative treatment for fear of the surgical bleeding risk. At present, there is no standardized and unified treatment standard for CHC, hepatic TACE is an effective treatment and targeted therapy combined with immunotherapy can be used as a clinical choice (11,12). Therefore, the patient received hepatic TACE therapy first, followed by Camrelizumab plus + Anlotinib Hydrochloride regimen for treatment (13). Aggressive surgical treatment was recommended for the patient, given that conservative treatment often leads to tumor progression, although the tumor treatment effect is PR according to mRECIST evaluation system. But the family members still chose conservative treatment, considering the good response to the drug. The postoperative pathological results showed that IHC was classified as IIIA stage according to the eighth edition AJCC staging of IHC. In order to prevent tumor recurrence, immunization and targeted therapy were continued one month after operation, and GemOx regimen chemotherapy was added (14). On discharge, the patients were told to continue adjuvant treatment and close follow-up. At present, the patient currently has an overall survival time of 18 months and is still followed-up.
Coagulation FV is synthesized in the liver, and FV deficiency may cause spontaneous or post-traumatic bleeding. Clinically, major hepatectomy has its own risk of bleeding (15), and moreover, coupled with the lack of FV will increase the risk of bleeding even life-threatening. These risks can be manifested of massive hemorrhage during hepatectomy, and the abnormal liver function aggravates the disturbance of coagulation factor synthesis after hepatectomy, which may induce massive hemorrhage in the surgical field. After accurate diagnosis by gene detection before operation, FFP was supplemented 2 days before operation, and the thrombin time was monitored in real time. Bleeding can be minimized by maintaining low central venous pressure (CVP), using Pringle maneuver to control inflow hepatic blood and fine operation during laparoscopic hepatectomy. After the operation, the patients were admitted to the intensive care unit for treatment, closely monitored the thrombin time and abdominal drainage, and supplemented with FFP if necessary. There were no serious complications during the perioperative period. Therefore, through the management of this patient, we believe that partial hepatectomy is safe and feasible for patients with liver disease complicated with FV deficiency, and this is the first reported case of partial hepatectomy for patients with FV deficiency. Several studies have proposed different standards for safe FV activity during surgical procedures. We believe that the safe level can be achieved by increasing FV activity to 20% during liver surgery.
We combined the MeSH words FV deficiency and hepatectomy to search in the PubMed database, and only one case report was found. The content of this case report was that a 67-year-old man underwent partial hepatectomy of S3 segment for recurrent hepatocellular carcinoma and was found to be complicated with deficiency of coagulation factor II and V after operation, which is also different from the massive hepatectomy mentioned in our case. There are some limitations. Firstly, our conclusion was only summarized from the experience of this case, which needs to be verified by more clinical cases. Secondly, the patient had received interventional therapy, targeted therapy and immunotherapy for liver cancer, and it is unknown whether it will affect the conclusion of the study, which needs to be further verified.
Of course, it is not clear whether the possibility of bleeding and the severity of bleeding may be related to the type of genetic variation, so we suggest that patients should be prepared for perioperative period as a condition of possible massive bleeding during the invasive operations, including preoperative fresh plasma infusion to improve FV activity and blood coagulation function (PT, APTT, etc.), adequate preparation of FFP and platelets during operation, careful operation to avoid bleeding, and close monitoring after the procedure.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-528/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-528/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-528/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol 2016;38:4-11. [Crossref] [PubMed]
- Tabibian S, Shiravand Y, Shams M, et al. A Comprehensive Overview of Coagulation Factor V and Congenital Factor V Deficiency. Semin Thromb Hemost 2019;45:523-43. [Crossref] [PubMed]
- Al-Numair NS, Ramzan K, Saleh M, et al. First description of the molecular and clinical characterization of hereditary factor V deficiency in Saudi Arabia: report of four novel mutations. Blood Coagul Fibrinolysis 2019;30:224-32. [Crossref] [PubMed]
- Tabibian S, Shiravand Y, Shams M, et al. A Comprehensive Overview of Coagulation Factor V and Congenital Factor V Deficiency. Semin Thromb Hemost 2019;45:523-43. [Crossref] [PubMed]
- Nuzzo F, Beshlawi I, Wali Y, et al. High incidence of intracranial bleeding in factor V-deficient patients with homozygous F5 splicing mutations. Br J Haematol 2017;179:163-6. [Crossref] [PubMed]
- Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors’ Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol 2014;167:304-26. [Crossref] [PubMed]
- Peyvandi F, Menegatti M. Treatment of rare factor deficiencies in 2016. Hematology Am Soc Hematol Educ Program 2016;2016:663-9. [Crossref] [PubMed]
- Meidert AS, Kinzinger J, Möhnle P, et al. Perioperative Management of a Patient with Severe Factor V Deficiency Presenting with Chronic Subdural Hematoma: A Clinical Report. World Neurosurg 2019;127:409-13. [Crossref] [PubMed]
- Gavva C, Yates SG, Rambally S, et al. Transfusion management of factor V deficiency: three case reports and review of the literature. Transfusion 2016;56:1745-9. [Crossref] [PubMed]
- Tao CY, Liu WR, Jin L, et al. Surgical Treatment of Combined Hepatocellular-Cholangiocarcinoma is as Effective in Elderly Patients as it is in Younger Patients: A Propensity Score Matching Analysis. J Cancer 2018;9:1106-12. [Crossref] [PubMed]
- Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma 2018;6:11-21. [Crossref] [PubMed]
- Schizas D, Mastoraki A, Routsi E, et al. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int 2020;19:515-23. [Crossref] [PubMed]
- Song F, Hu B, Cheng JW, et al. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis 2020;11:573. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Prodeau M, Drumez E, Duhamel A, et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol 2019;71:920-9. [Crossref] [PubMed]


