
Efficacy of endoscopic resection and intensity-modulated radiotherapy for thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report
Introduction
Malignant carcinomas in the nasopharynx originate from the nasopharyngeal mucosal epithelium, with those exhibiting squamous differentiation under optical and electron microscopy being the most common. Although this diagnostic term sounds relatively nonspecific, nasopharyngeal carcinoma only refers to squamous cell carcinomas (SCCs) and not adenocarcinoma or carcinoma arising from the salivary gland: the latter 2 account for less than 5% of all malignant nasopharyngeal tumors. Low-grade nasopharyngeal papillary adenocarcinoma (LGNPPA) is extremely rare among nasopharyngeal neoplasms. To our knowledge, fewer than 70 cases have been publicly reported thus far (1-19). Because of the very low incidence of this disease, there are no guidelines or protocols developed for proper, standardized therapy. Most scholars suggest surgery for patients at early disease stages and radiotherapy for patients with residual tumors. A case report of a patient free of recurrence and metastasis at 15 years of follow-up has been published (12). The prognosis is good, with recurrence and metastasis being rare. Because there are few reports about the disease both at domestic and abroad and mainly focus on the clinicopathological features. Treatment methods, side effects, quality of life and follow-up were rarely reported. Herein, we report a case of LGNPPA in which the patient was followed up for more than 6 years after intensity-modulated radiotherapy and reported a good quality of life. This study explores the diagnosis, therapy, and follow-up pertaining to the therapeutic effects and side effects of this case, and may thus serve as an important reference for the treatment of this rare carcinoma subtype. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1166/rc).
Case presentation
History of recent illness
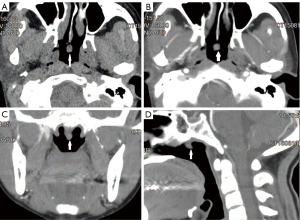
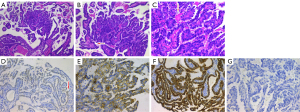
A 45-year-old female of Han ethnicity visited a local hospital for over 2 years of recurrent nasal congestion. On August 2015, computed tomography (CT) scans of the nasal cavity and paranasal sinuses of the patient conducted in her local hospital showed a tumor located in the upper nasopharynx (Figure 1). Nasal endoscopy revealed the following: (I) hypertrophic rhinitis, (II) nasal septal deviation, and (III) a neoplasm in the upper nasopharynx (Figures 1-3). The patient underwent nasal septal deviation correction + bilateral middle and inferior turbinate resection + nasopharyngeal neoplasm removal under local anesthesia at the local hospital on August 11, 2015. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The pathology department of Affiliated Hospital of Zunyi Medical University was consulted for tumor section analyses (H15347, Figure 2), and the results indicated LGNPPA. The immunohistochemical staining results were as follows: cytokeratin (CK+), CK19+, CK20−, CK7+, CK8+, epithelial membrane antigen (EMA+), thyroglobulin (TG), transcription termination factor 1 (TTF1+), and vimentin+; additionally, the sample was periodic acid-Schiff (PAS+). The patient was transferred to our hospital for further therapy on August 19, 2015.
History of past illness
The patient had no previous medical history. The patient had a history of smoking (6 years, 20 cigarettes per day) and drank occasionally, but had no family history of cancer.
Physical examination
An examination by a specialist indicated that the patient’s olfactory function was intact and that the bilateral cervical lymph nodes were impalpable; additional tests on 12 pairs of cranial nerves were negative.
Laboratory examination
Routine blood and biochemical tests were normal. Epstein-Barr virus (EBV) DNA was <500×102 IU/mL.
Imaging examinations
Chest CT, abdominal color Doppler ultrasound, emission CT (ECT), and electrocardiography results were all normal. Plain and enhanced magnetic resonance imaging (MRI) of the nasopharynx and neck revealed no thickening of the nasopharyngeal mucosa, no enhancement on the enhanced scan, and no narrowing of the bilateral pharyngeal recesses. The morphology of the parapharyngeal space was normal, but multiple small lymph nodes were observed on both sides of the neck.
Final diagnosis
The final diagnosis of the presented case was low-grade papillary adenocarcinoma in the upper nasopharynx (T1N0M0, stage I).
Treatment
After undergoing nasopharyngeal neoplasm removal at the local county hospital, the patient was transferred to our hospital for intensity-modulated radiotherapy. After her eligibility was verified, intensity-modulated radiotherapy was performed from September 11, 2015, to November 2, 2015, with a dose of 95% PGTVnx 73.92 Gy/2.24 Gy/33 f, 95% PTV1 66 Gy/2 Gy/33 f, and 95% PTV2 56.42 Gy/1.82 Gy/28 f. During the radiotherapy, moist desquamation developed but resolved after treatment.
Outcome and follow-up
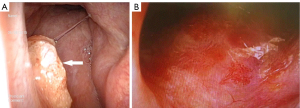
After the therapy, nasopharynx and neck MRI (plain scan + enhanced), nasopharyngeal endoscopy, chest CT, abdominal color Doppler ultrasound, and EBV DNA testing were performed every 3 months in the first 2 years and then every 6 months until the end of the 72 months of follow-up. No local residual tumor, tumor recurrence, or distant metastasis was found under nasopharyngeal endoscopy (Figures 2 and 3) in the last follow-up in December 2021. The therapeutic effect was evaluated as complete remission.
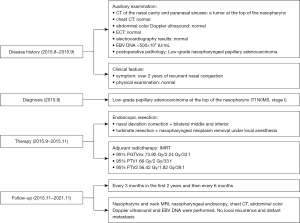
The timeline of disease history, diagnosis, therapy, and follow-up outcomes has been summarized (Figure 4).
Discussion
Among the malignant tumors that occur in the nasopharynx, nasopharyngeal carcinoma is the most common. Nasopharyngeal carcinoma is a relatively nonspecific term and refers to all nasopharyngeal SCCs (20). Thus, primary nasopharyngeal adenocarcinomas (NPACs) are not classified as nasopharyngeal carcinomas. NPACs are very rare, accounting for 0.3–0.48% of nasopharyngeal malignant tumors in the nasopharynx (21-23). Thyroid-like LGNPPAs (TL-LGNPPAs) constitute a special subtype of NPACs; therefore, the incidence of TL-LGNPPA is even more rare. As the name implies, TL-LGNPPA is an adenocarcinoma that originates from the nasopharynx; it has a papillary adenoid structure, grows exogenously and invasively, appears immunohistochemically TTF-1 positive, and is often low grade. In a retrospective study from 1988, TL-LGNPPA was first reported as a separate solid tumor (24). In 2005, Carrizo et al. (12) reported 2 cases of LGNPPA with an immunohistochemistry of TTF-1+ and TG−, and were the first to refer to this as TL-LGNPPA, which remains the accepted term today. In these cases, the tumor arose from the upper nasopharynx; under light microscopy, the tumor cells were arranged as a papillary structure with a fibrovascular axis inside and formed a single layer with cleat-like protrusions. The immunohistochemical staining results were as follows: CK7+, TTF-1+, CK19+, and TG−. The neck MRI scan showed no space-occupying lesions, which is consistent with a diagnosis of TL-LGNPPA.
LGNPPA is so rare that few reports—either in China or internationally—exist regarding this disease, with those that have been published being mainly focused on clinical and pathological characteristics, with limited content regarding therapy, side effects, and follow-up. Most studies show that surgical resection, especially the endoscopic resection of lesions, is an effective treatment (1-4,25,26); if surgery is not practical or the surgical margin is positive, postoperative radiotherapy is often conducted to prevent recurrence (6-8). Due to the small number of cases, there are currently no guidelines or protocols to direct treatment; thus, a standardized and optimal treatment strategy is unavailable.
If resection is feasible, surgery is the preferred treatment for adenocarcinoma (25). Because the nasopharynx is a very complex structure—located in the center of the skull base and surrounded by a series of important organs (such as the brain stem, spinal cord, and cranial nerves)—open surgery to access the nasopharynx is very challenging. Specifically, it is destructive to the face and surrounding vital organs, is associated with many complications, and may lead to incomplete removal of the tumor. Endoscopic resection or endoscopic resection-based therapy may be an effective method, especially for small tumors (25,26). Takakura et al. reported a case of LGNPPA free of local recurrence and distant metastasis for 5 years after endoscopic resection, thus concluding that patients with LGNPPA have a very good prognosis and that complete resection with an adequate safety margin should the first-line treatment (2). In a recent single-center, retrospective study (1), 23 out of 28 patients included in the study underwent pure endoscopic treatment, with 2 cases of recurrence in the following 7–121 months of follow-up and 0 cases of recurrence or distant metastasis after endoscopic resection was applied a second time for tumor recurrence. LGNPPA is believed to be an inert cancer with a very good prognosis, for which endoscopic resection is an effective treatment.
Whether radiotherapy can also be used in some cases of LGNPPAs should be investigated. The special anatomical location of the nasopharynx and its adjacency to vital organs make surgical resection difficult, and thus the effects of radiotherapy for the treatment of LGNPPA should be further studied. Wang et al. published a case report of a patient free of local recurrence and distant metastasis after postoperative photodynamic therapy for nasopharyngeal papillary adenocarcinoma (27). Additionally, in a retrospective study of 28 patients by Lai et al. (1), 3 patients underwent preoperative radiotherapy (the sizes of the tumors were significantly reduced), 2 patients underwent postoperative adjunct radiotherapy, and 23 patients underwent pure endoscopic surgery; in the follow-up, 5 patients who received radiotherapy were alive without recurrence and metastasis, and 2 patients who had pure endoscopic surgery experienced recurrence. These results indicate that tumor cells may react to radiotherapy and that combined radiotherapy can help to increase disease-free survival. Thus, some researchers believe that surgery combined with radiotherapy may be the proper treatment strategy for limited or resectable low-grade NPACs (28) and that postoperative adjuvant radiotherapy is an effective way to prevent recurrence, especially for unresectable tumors or tumors with a suspected positive margin (27). However, due to the limited number of patients reported in the literature, further studies are needed to elucidate the role of radiotherapy for the treatment of LGNPPA.
In the present case, the patient underwent neoplasm removal surgery under local anesthesia at the local hospital. As the lesion was mistakenly identified as a benign tumor under endoscopy, therapy focused on correction of the nasal septum to alleviate nasal congestion rather than on cancer therapy; thus, only endoscopic tumor removal was performed. After the diagnosis of LGNPPA was confirmed by pathology, the patient was transferred to the central hospital for further therapy. Based on the initial endoscopic and pathological findings and examinations performed after admission to our hospital, the patient was diagnosed with low-differentiated nasopharyngeal papillary adenocarcinoma (T1N0M0/stage I). Although the stage was early and the pathological grade was low, tumor recurrence could not be avoided. The local hospital had little experience in oncology due to limited expertise in diagnosis and treatment. Only tumor removal with a limited surgical scope was performed; it was impossible to ensure that the tumor had been completely removed or that the surgical margin was microscopically negative. Clinically, reoperation or postoperative radiotherapy is often applied for tumors with positive or unknown margins. Although LGNPPA grows slowly, incomplete resection can still lead to recurrence, and thus radiotherapy should be applied to treat potential residual tumors (5). In addition, LGNPPA is a highly differentiated adenocarcinoma with low sensitivity to conventional radiotherapy or chemoradiotherapy (28). Considering all these factors, we administered weekly cisplatin (50 mg per cycle) with concurrent intensity-modulated radiotherapy at a dose higher than that for poorly differentiated SCC. Due to a severe gastrointestinal reaction, cisplatin was discontinued after the fifth cycle of chemotherapy. During the entire radiotherapy period, the patient experienced I° bone marrow suppression, a mild oral mucosal reaction, and II° radiodermatitis in the neck, which resolved after treatment. The whole radiotherapy process was smooth. In the 62 months of follow-up, the patient experienced only pigmentation in the skin behind the ears and mild dry mouth, without serious late-stage side effects of radiotherapy, such as neck skin fibrosis and fat tissue loss, temporomandibular joint dysfunction, hearing damage, cranial nerve damage, and temporal lobe necrosis. Regular EBV DNA tests were negative, and neither recurrence in the primary location and regional lymph nodes nor distant metastasis was observed in endoscopic and imaging examinations. The patient had a good prognosis.
The positive therapeutical outcome in this case was due to the following reasons. (I) Physicians in primary hospitals have accumulated a certain amount of theoretical knowledge about cancer. Once the diagnosis of malignant tumor was made, the physicians, without delay, transferred the patient to a hospital that specializes in oncology, realizing their limited experience in diagnosing and treating cancer. (II) Based on the surgical method that was initially used in the local hospital and the biological characteristics of poorly differentiated adenocarcinomas (e.g., reduced sensitivity to chemoradiotherapy compared to poorly differentiated squamous cancer), concurrent postoperative chemoradiotherapy was adopted after a comprehensive evaluation. (III) Standardized target area delineation ensured that the dose was effectively delivered to the tumor but strictly controlled to avoid organ damage, thus improving and guaranteeing the quality of life of the patient now and in the future. (IV) The acute mucosal and skin reactions that occurred during therapy were addressed effectively and in a timely manner, thereby preventing the development of chronic side effects.
As LGNPPA is extremely rare, there is no standard treatment strategy. Most patients are treated with surgery, after which a negative surgical margin must be ensured. Postoperative radiotherapy is used in some cases with positive or unknown margins. Through observation of the therapy process, treatment strategy, and long-term therapeutic outcomes, the patient achieved complete clinical remission and a good quality of life without serious late-stage side effects. This case report has been published to serve as a reference for the treatment strategy for this disease. Because reports of this disease are mostly individual cases, it is necessary to carry out large-scale multicenter studies or a meta-analysis to further evaluate the best treatment strategy and the most strongly related prognostic factors to improve the quality of life of and reduce the mental and economic burden on patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1166/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1166/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai Y, Li W, Zhai C, et al. Low-Grade Nasopharyngeal Papillary Adenocarcinoma: A Review of 28 Patients in a Single Institution. Cancer Manag Res 2021;13:1271-8. [Crossref] [PubMed]
- Takakura H, Hamashima T, Tachino H, et al. Clinicopathological Features of Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma: A Case Report and Review of the Literature. Front Surg 2020;7:596796. [Crossref] [PubMed]
- Maocai L, Fuxing L, Lianqing L, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: A case report. Medicine (Baltimore) 2020;99:e21599. [Crossref] [PubMed]
- Zhang WL, Ma S, Havrilla L, et al. Primary thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: A case report and literature review. Medicine (Baltimore) 2017;96:e8851. [Crossref] [PubMed]
- Malik L, Hemmings C, Beshay V, et al. Metastatic gastrointestinal stromal tumour of the ileum with dual primary c-KIT missence mutations. Pathology 2013;45:604-6. [Crossref] [PubMed]
- Huang F, Xiang X, Hong B, et al. Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma. Am J Clin Pathol 2019;152:582-9. [Crossref] [PubMed]
- Petersson F, Pang B, Loke D, et al. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: report of a case localized to the posterior nasal septum. Head Neck Pathol 2011;5:306-13. [Crossref] [PubMed]
- Ohe C, Sakaida N, Tadokoro C, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int 2010;60:107-11. [Crossref] [PubMed]
- Bansal A, Pradeep KE, Gumparthy KP. An unusual case of low-grade tubulopapillary adenocarcinoma of the sinonasal tract. World J Surg Oncol 2008;6:54. [Crossref] [PubMed]
- Wu PY, Huang CC, Chen HK, et al. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg 2007;137:837-8. [Crossref] [PubMed]
- Pineda-Daboin K, Neto A, Ochoa-Perez V, et al. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol 2006;10:215-21. [Crossref] [PubMed]
- Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol 2005;9:189-92. [Crossref] [PubMed]
- Li L, Zhou F, Lin F, et al. Clinicopathologic Characteristics of Thyroid-like Low-grade Nasopharyngeal Papillary Adenocarcinoma: A Case Report. Appl Immunohistochem Mol Morphol 2019;27:e81-4. [Crossref] [PubMed]
- Li M, Wei J, Yao X, et al. Clinicopathological Features of Low-Grade Thyroid-like Nasopharyngeal Papillary Adenocarcinoma. Cancer Res Treat 2017;49:213-8. [Crossref] [PubMed]
- Ozturk K, Midilli R, Veral A, et al. Primary thyroid-like papillary adenocarcinoma of the nasal septum: a case report. Ear Nose Throat J 2015;94:E19-21. [Crossref] [PubMed]
- Huang CH, Chang YL, Wang CP, et al. Positive immunostaining of thyroid transcription factor-1 in primary nasopharyngeal papillary adenocarcinoma. J Formos Med Assoc 2015;114:473-4. [Crossref] [PubMed]
- Oishi N, Kondo T, Nakazawa T, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: case report and literature review. Pathol Res Pract 2014;210:1142-5. [Crossref] [PubMed]
- Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19-year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e25-8. [Crossref] [PubMed]
- Fu CH, Chang KP, Ueng SH, et al. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx 2008;35:579-82. [Crossref] [PubMed]
- Stelow EB, Wenig BM. Update From The 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Nasopharynx. Head Neck Pathol 2017;11:16-22.
- Guo ZM, Liu WW, He JH. A retrospective cohort study of nasopharyngeal adenocarcinoma: a rare histological type of nasopharyngeal cancer. Clin Otolaryngol 2009;34:322-7. [Crossref] [PubMed]
- Liu LZ, Zhang YM, Chen Y, et al. Spreading patterns, prognostic factors and treatment outcomes of nasopharyngeal papillary adenocarcinoma and salivary gland-type carcinomas. Clin Otolaryngol 2016;41:160-8. [Crossref] [PubMed]
- He JH, Zong YS, Luo RZ, et al. Clinicopathological characteristics of primary nasopharyngeal adenocarcinoma. Ai Zheng 2003;22:753-7. [PubMed]
- Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma. A clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol 1988;12:946-53. [Crossref] [PubMed]
- Ünsaler S, Başaran B, Aslan I, et al. Endonasal endoscopic nasopharyngectomy for the treatment of nasopharyngeal papillary adenocarcinoma: Report of a rare case. Int J Pediatr Otorhinolaryngol 2018;104:51-3. [Crossref] [PubMed]
- Ryu J, Park WS, Jung YS. Exclusive Endoscopic Resection of Nasopharyngeal Papillary Adenocarcinoma via Combined Transnasal and Transoral Approach. Clin Exp Otorhinolaryngol 2013;6:48-51. [Crossref] [PubMed]
- Wang CP, Chang YL, Chen CT, et al. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med 2006;38:435-8. [Crossref] [PubMed]
- Wang X, Yan H, Luo Y, et al. Low-grade nasopharyngeal papillary adenocarcinoma: a case report and review of the literature. Onco Targets Ther 2016;9:2955-9. [PubMed]
(English Language Editor: J. Gray)


