
Associations of CD34, Ki67, layer of invasion and clinical pathological characteristics, prognosis outcomes in gastrointestinal stromal tumors—a retrospective cohort study
Introduction
Gastrointestinal stromal tumor (GIST) is a rare gastrointestinal tumor with an annual global incidence of 1–2 per 100,000. A study has shown an incidence of 2.11 per 100,000 in Shanghai, China (1), and an incidence of 1.8 per 100,000 in Italy (2). GIST is the most common interstitial tumor of the digestive tract (3), which occurs mostly in organs of the digestive tract system such as the stomach (the most common site), small intestine, colorectum, and peritoneum (4). Before 2000, due to the lack of clarity about the molecular mechanism of GIST, our understanding of GIST was insufficient, the treatment methods were relatively few, and the overall prognosis was poor (5). Like other malignancies, GIST can recur, metastasize, and even metastasize to the brain, resulting in death (6). In recent years, with the progress of life sciences, the molecular mechanism of GIST has gradually been recognized, the application of targeted therapy under the guidance of genetic testing in GIST has been promoted, the prognosis of GIST has been greatly improved, the importance of genetic testing has been recognized, and it has played an important role in guiding GIST treatment (7,8). However, in the real world, especially in Asia (9), due to economic and other reasons, genetic testing and drug use may not be readily suitable for clinical trials, especially among low-risk patients, who are reluctant to undergo expensive genetic testing, and even some medium- and high-risk patients who refuse genetic testing and related drug treatment after surgery. In addition to commonly used treatments, including surgery and targeted therapy, the use of radiotherapy in GIST is also being explored (10), and the correlation between treatment and prognosis has also been studied (11).
In recent years, with the development of endoscopic technology, the diagnosis and treatment of GIST has also improved. A Japanese study by Akahoshi et al. showed that endoscopic technology played an important role in the early management of GIST (12), and the overall diagnostic rate of submucosal tumors has been shown to be 62.0–93.4% (13,14). Although surgery was previously taken as the main means of pathological investigation, endoscopic technology is more conducive to achieving a comprehensive understanding of the changes in clinical characteristics and immune indicators during the development of GIST and their correlations. At present, there is a lack of authoritative biomarkers to predict the prognosis of GIST patients. To date, the role of risk classification in the development of GIST has been clear (15), but the significance of Cluster Differentiation 34 (CD34) and Ki67 in GIST has remained controversial (16-18), and the layer of invasion of GIST has rarely been studied. But with advances in endoscopic technology in recent years, there have been more and more cases of endoscopic submucosal dissection (ESD). To gain a more comprehensive understanding of GIST and studied the factors affecting the prognosis, we included cases of combined targeted therapy with ESD surgery or surgery, in order to study its clinical features, immune indicators, and their correlations, and to analyze their role in GIST and their relationship to prognosis. We present the following article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1777/rc).
Methods
Patient selection
From 2013 to June 2020, 878 patients from the Northern Jiangsu People’s Hospital in Yangzhou were selected. A total of 455 cases of GIST were retrospectively enrolled, and the remaining 423 cases were screened according to the exclusion criteria. All patients had confirmed pathologies (including very low-risk, low-risk, medium-risk, and high-risk patients). The exclusion criteria were as follows: (I) concomitant malignancy that affected the progression-free survival (PFS) or overall survival (OS); (II) lost to follow-up; and (III) death within one month after surgery. Among them, 73 cases were treated with ESD, 273 cases were treated with surgery, and 77 cases were treated with surgery and imatinib. The data were derived from patients’ case records, or subsequent follow-up reports. There were a few patients for whom individual information was missing, which will be shown in subsequent charts, and all patient information was anonymized. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by the Ethics committee of Northern Jiangsu People’s Hospital due to the following reasons: (I) this study was a retrospective observational study; only the clinical data of the patients were analyzed, which could not negatively impact the patients. (II) The authors will protect the information provided by patients from encroaching on their personal privacy. Individual consent for this retrospective analysis was waived.
The last follow-up time was in August 2021, and the survival time was from onset to death or the last follow-up.
Evaluation criteria
Evaluate the risk of the patients according to the improved grading criteria of the National Institutes of Health (NIH; Bethesda, MD, USA) (tumor size, primary site, nuclear division, and rupture). Patient progression, including recurrence and metastasis, was confirmed by gastroscopy or CT.
Immunohistochemistry (IHC)
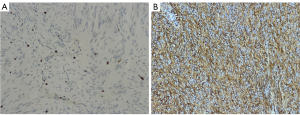
During surgery, patients’ pathological specimens were obtained, and the enzyme chain immunoassay was used to analyze the expression of CD34 and Ki67 in the tumor tissue. After the specimen was fixed, it was made into wax blocks (stored at room temperature and protected from light), and then unified into 5-µm thick specimens and placed on slides. The CD34 and Ki67 staining was processed by an automated staining instrument (Wentana Medical Systems, Tucson, AZ, USA) (Figure 1).
Statistical analysis
The software SPSS 24.0 (IBM Corp., Armonk, NY, USA) was used for statistics, and P<0.05 indicated a statistical difference (two-sided). The associations between CD34, Ki67, layer of invasion and clinical pathological characteristics in GIST were analyzed using chi-square test; the association between the layer of invasion and Ki67 was analyzed using chi-square test; and the associations between CD34, Ki67, layer of invasion and prognosis were analyzed Kaplan-Meier (KM) survival curves.
Results
General condition of the patients
A total of 423 cases were enrolled, including 202 males and 221 females. Their average age was 60.59±10.19 years (aged from 30 to 87 years), the median age was 61 years (Figure 2).
Correlation of CD34 with clinical features, PFS, and OS
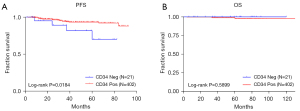
Among the 423 patients, 21 cases were CD34 negative, and 402 cases were positive. Our study found that CD34 was associated with clinical features such as primary site, tumor size, risk, and recurrence, and had no significant correlation with nuclear division (Table 1). Although CD34 had a significant correlation with PFS, it was not significantly correlated with OS (Figure 3).
Table 1
| Variables | CD34, n | χ2 | P value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Primary site | 57.496 | <0.001 | ||
| Stomach | 2 | 335 | ||
| Small intestine | 15 | 36 | ||
| Colon/rectum | 1 | 14 | ||
| Peritoneum | 3 | 17 | ||
| Tumor size (cm) | 14.983 | <0.001 | ||
| ≤2 | 0 | 119 | ||
| >2 and ≤5 | 10 | 142 | ||
| >5 and ≤10 | 5 | 101 | ||
| >10 | 6 | 40 | ||
| Risk level | 17.788 | <0.001 | ||
| Very low | 0 | 116 | ||
| Low | 8 | 130 | ||
| Intermediate | 2 | 79 | ||
| High | 11 | 77 | ||
| Relapsed or not | 4.239 | 0.039 | ||
| Relapsed | 4 | 22 | ||
| Not relapsed | 17 | 380 | ||
| Mitotic index (HPF) | 1.030 | 0.310 | ||
| ≤5/50 | 16 | 348 | ||
| >5/50 | 5 | 54 | ||
CD34, Cluster Differentiation 34; HPF, high power field.
Correlation between Ki67 and clinical features, PFS, and OS
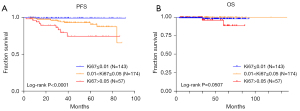
In 423 cases, 374 patients had the value of Ki67 detected in tumor tissue, and Ki67 was shown to be associated with nuclear division, tumor size, risk, and recurrence (Table 2). It was shown that Ki67 had a correlation with PFS (P<0.05) and some correlation with OS, although not statistically significant (P>0.05) (Figure 4).
Table 2
| Variables | Ki67, n | χ2 | P value | ||
|---|---|---|---|---|---|
| ≤0.01 | >0.01 and ≤0.05 | >0.05 | |||
| Mitotic index (HPF) | 61.878 | <0.001 | |||
| ≤5/50 | 141 | 155 | 31 | ||
| >5/50 | 2 | 19 | 26 | ||
| Tumor size (cm) | 32.457 | <0.001 | |||
| ≤2 | 58 | 45 | 6 | ||
| >2 and ≤5 | 46 | 66 | 20 | ||
| >5 and ≤10 | 33 | 43 | 16 | ||
| >10 | 6 | 20 | 15 | ||
| Risk level | 39.96 | <0.001 | |||
| Very low | 56 | 45 | 5 | ||
| Low | 46 | 61 | 14 | ||
| Intermediate | 27 | 32 | 12 | ||
| High | 14 | 36 | 26 | ||
| Relapsed or not | 17.504 | <0.001 | |||
| Relapsed | 1 | 12 | 9 | ||
| Not relapsed | 142 | 162 | 48 | ||
HPF, high power field.
Correlation between the layer of invasion and clinical features, PFS, and OS
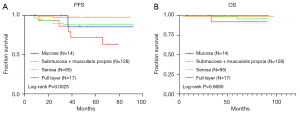
Of the 423 patients, a total of 244 had data of the layer of invasion, which was found to be associated with primary site, nuclear division, tumor size, risk, CD34, smooth muscle actin (SMA), and recurrence (Table 3). The layer of invasion was also associated with PFS (P=0.0025), but not significantly with OS (P=0.6680) (Figure 5).
Table 3
| Variables | The layer of invasion, n | χ2 | P value | |||
|---|---|---|---|---|---|---|
| Mucosa | Submucosa + muscularis propria | Serosa | Full layer | |||
| Primary site | 37.726 | <0.001 | ||||
| Stomach | 11 | 116 | 57 | 7 | ||
| Small intestine | 2 | 8 | 21 | 9 | ||
| Colon/rectum | 1 | 4 | 4 | 0 | ||
| Peritoneum | 0 | 0 | 3 | 1 | ||
| Mitotic index (HPF) | 13.775 | <0.001 | ||||
| ≤5/50 | 10 | 118 | 69 | 11 | ||
| >5/50 | 4 | 10 | 16 | 6 | ||
| Tumor size (cm) | 45.3 | <0.001 | ||||
| ≤2 | 0 | 39 | 5 | 1 | ||
| >2 and ≤5 | 7 | 54 | 27 | 7 | ||
| >5 and ≤10 | 5 | 29 | 35 | 4 | ||
| >10 | 2 | 6 | 18 | 5 | ||
| Risk level | 52.821 | <0.001 | ||||
| Very low | 0 | 39 | 5 | 1 | ||
| Low | 5 | 52 | 25 | 5 | ||
| Intermediate | 4 | 25 | 21 | 2 | ||
| High | 5 | 12 | 34 | 9 | ||
| Relapsed or not | 17.946 | <0.001 | ||||
| Relapsed | 1 | 2 | 9 | 5 | ||
| Not relapsed | 13 | 126 | 76 | 12 | ||
| CD34 | 13.795 | <0.001 | ||||
| Negative | 0 | 3 | 5 | 5 | ||
| Positive | 14 | 125 | 80 | 12 | ||
| SMA | 11.075 | <0.001 | ||||
| Negative | 12 | 121 | 68 | 16 | ||
| Positive | 2 | 7 | 17 | 1 | ||
SMA, smooth muscle actin; CD34, Cluster Differentiation 34; HPF, high power field.
Relevance of the layer of invasion to Ki67
Of the 423 patients, there were data for both Ki67 and the layer of invasion for a total of 223 cases, and we found a clear correlation between them (Table 4).
Table 4
| Variables | The layer of invasion, n | χ2 | P value | |||
|---|---|---|---|---|---|---|
| Mucosa | Submucosa + muscularis propria | Serosa | Full layer | |||
| Ki67 | 16.558 | <0.01 | ||||
| ≤0.01 | 1 | 43 | 24 | 6 | ||
| >0.01 and ≤0.05 | 4 | 63 | 37 | 5 | ||
| >0.05 | 7 | 14 | 14 | 5 | ||
Discussion
Through the study of GIST cases in Northern Jiangsu People’s Hospital, it was found that the number of male cases is slightly lower than that of females (202/221), which is slightly different to a previous study (2). The average age of the patients in this study was 60.69 years, and the median was 61 years, which was different from the studies of Liu et al. and Alghamdi et al. (16,19) wherein the average age was 54–58 years. Their patients were from Italy, Saudi Arabia, or Guangdong Province, China, and our study patients were mainly from the Jiangsu Province, China, indicating that the gender and age of GIST still varies from region to region.
Although Magnetic resonance imaging has been widely used in the diagnosis and treatment of cancer (20), the effect of Magnetic resonance imaging in differentiating the benign and malignant GIST is poor. IHC is key to the diagnosis of GIST, and a study has suggested that immune indicators play an important role in the prognosis of GIST (21). We found that CD34 was closely related to the clinical features and prognosis of GIST, which is consistent with the study by Miettinen et al. (22). However, some studies have concluded that CD34 is not associated with the prognosis of GIST (23,24). In our study, the overall negative rate for CD34 was 4.96%, but in a previous study, the negative rate for CD34 was 20–40% (9). We found that the negative rate was as high as 22.09% in non-gastric tumors, and the negative rate from gastric sources was 0.59%. A study has shown that different sources of stromal tumors have different biological characteristics (25), and that lesions derived from the stomach have a better prognosis (26). Another study showed that CD34 has a high negative rate in large lesions, with the highest negative rate (13.04%) in the >10 cm group and the lowest negative rate (0%) in the ≤2 cm group (9). There is a consensus that tumors smaller than 2 cm can be left untreated and observed regularly (9), and a study concluded that very small GIST has a very low likelihood of malignancy (27). The risk level classification is an internationally recognized prognostic standard. In high-risk patients, the CD34 negative rate is 12.50%. In very low-risk patients, the CD34 negative rate was 0%. In the relapsed case group, the CD34 negative rate was 15.38%, and in the non-recurrence group, the CD34 negative rate was 4.28%. A previous study showed that positive CD34 may be an unfavorable prognostic factor for GIST (28), but in our study, the positive CD34 group had a longer PFS. The above results showed that CD34 played a certain role in the occurrence and development of GIST, and the increase in its negative rate was an unfavorable factor for the prognosis of GIST, but it had no obvious significance in terms of OS.
The degree of malignancy of a tumor is correlated with its cell growth activity (29). Ki67 is an associated antigen with cell cleavage and is closely related to mitosis, and has been found to be a potential prognostic factor for GIST (30). This study found that Ki67 was associated with several clinical features, including nuclear division, tumor size, risk, and recurrence or not. Ki67 has a clear correlation with internationally recognized risk levels, and it has been reported that Ki67 is strongly associated with tumor sources (31), which was not found in our study. The results of this study suggested that there are significant differences in PFS in patients with different stratifications of Ki67, indicating that Ki67 is of great significance in predicting the development of GIST before recurrence. From this study, we inferred that Ki67 also plays an important role in the occurrence and expansion of GIST, and that higher Ki67 is an unfavorable factor in the prognosis of GIST, which was similar to a previous study (32).
The layer of invasion indicates the growth direction and state of the tumor, which is an important clinical indicator. Endoscopy plays an important role in the management of early GIST (12), and with the popularization of endoscopic use, we have a better understanding of the layer of invasion in GIST. Our study showed that the layer of invasion was associated with multiple clinical features and immune markers; the layer of invasion varied markedly from different sources. A study showed that cases originating in the small intestine were significantly more susceptible to serous membrane (21/40) and full-thickness (9/40) invasion, and GIST derived from the small intestine had a worse prognosis than those from the stomach (33), which is consistent with our study. We found that in the full-thickness invasion group, the rate of nucleus division >5/50 was 35.29%; in the mucosal layer invasion group, the rate of nucleus division >5/50 was 28.57%; in the serous layer invasion group, the rate of nucleus division >5/50 was 18.82%; and in the submucosa and muscle invasion group, the rate of nucleus division >5/50 was 7.8%.
When the tumor size ≤2 cm, 86.67% cases had only invaded the submucosal and muscular layers; when the lesion was >2 and ≤5 cm, 56.84% patients had only invaded the submucosal and muscular layers. When the tumor size >5 and ≤10 cm, the most invaded layer was the serous layer (47.95%); when the tumor size >10 cm, the most invaded layer was also the serous layer (58.06%). This indicates that the larger the tumor, the deeper the layer of invasion. This intuitively allows us to recognize the trend of GIST growth. Among patients with recurrence, we found that the recurrence rate of invasion of the full layer was the highest (29.41%), followed by invasion of the serous layer (10.59%).
We also found that there was a correlation between the layer of invasion and CD34 and SMA. The CD34 negative rate (29.41%) was highest in the patients with full layer invasion, and the SMA expression of those with different invasion layer was different, which is worth further exploration. At the same time, the layer of invasion of GIST has obvious correlation with PFS and no obvious correlation with OS. In addition, we also found that Ki67 had a clear correlation with the layer of invasion: in the group of invasions of the mucosal layer, Ki67 >0.05 accounted for the highest proportion of 58.33%, followed by the full layer invasion group (31.25%), which notably suggested that the tumor activity of GIST that violated the mucosal layer is large. These findings suggest that the layer of invasion is an important factor influencing the prognosis of GIST, especially in patients with full-thickness invasion who are at higher risk and more prone to recurrence.
Risk classification is a recognized prognostic indicator of GIST, and previous studies have reported that the prognosis of GIST may be related to CD34 and Ki67 (22,30,32). However, there have also been reports of no correlation between CD34 and the prognosis of GIST (23,24). There are currently no clear reports on the layer of invasion and the prognosis of GIST. Our research suggests that CD34, Ki67, and the layer of invasion may be important factors affecting the development and prognosis of GIST, and for the first time, we found that layer of invasion was associated with GIST prognosis. However, due to the influence of pathological outcome data and the limited number of cases, some indicators have a certain correlation with prognosis, but they are not statistically significant. Our next step is to continue to expand the sample size and conduct more in-depth research.
Our study confirmed the correlation between the relevant indicators and prognosis of GIST and found that they could serve as new prognostic factors of the GIST, guiding the prognostic assessment of the GIST. With further research on GIST worldwide, we will find more and more clinical features and immune indicators that influence the prognosis of this disease, so as to guide the treatment and improve the outcome in GIST patients. The limitations of the present study was that it was a retrospective study and some data of the patients was missing.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1777/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1777/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1777/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by the Ethics committee of Northern Jiangsu People’s Hospital due to the following reasons: (I) This study was a retrospective observational study; only the clinical data of the patients were analyzed, which could not negatively impact the patients. (II) The authors will protect the information provided by patients from encroaching on their personal privacy. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lv M, Wu C, Zheng Y, et al. Incidence and survival analysis of gastrointestinal stromal tumors in shanghai: a population-based study from 2001 to 2010. Gastroenterol Res Pract 2014;2014:834136. [Crossref] [PubMed]
- Cuccaro F, Burgio Lo Monaco MG, Rashid I, et al. Population-based incidence of gastrointestinal stromal tumors in Puglia. Tumori 2021;107:39-45. [Crossref] [PubMed]
- von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol 2018;36:136-43. [Crossref] [PubMed]
- Siow SL, Mahendran HA, Wong CM. Laparoscopic transgastric resection for intraluminal gastric gastrointestinal stromal tumors located at the posterior wall and near the gastroesophageal junction. Asian J Surg 2017;40:407-14. [Crossref] [PubMed]
- Iwatsuki M, Harada K, Iwagami S, et al. Neoadjuvant and adjuvant therapy for gastrointestinal stromal tumors. Ann Gastroenterol Surg 2018;3:43-9. [Crossref] [PubMed]
- Prablek M, Srinivasan VM, Srivatsan A, et al. Gastrointestinal stromal tumor with intracranial metastasis: case presentation and systematic review of literature. BMC Cancer 2019;19:1119. [Crossref] [PubMed]
- Wang Y, Call J. Mutational Testing in Gastrointestinal Stromal Tumor. Curr Cancer Drug Targets 2019;19:688-97. [Crossref] [PubMed]
- Judson I, Bulusu R, Seddon B, et al. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res 2017;7:6. [Crossref] [PubMed]
- Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155-66. [Crossref] [PubMed]
- Cannella R, La Grutta L, Midiri M, et al. New advances in radiomics of gastrointestinal stromal tumors. World J Gastroenterol 2020;26:4729-38. [Crossref] [PubMed]
- Pan F, Den J, Zhang C, et al. The Therapeutic Response of Gastrointestinal Stromal Tumors to Imatinib Treatment Assessed by Intravoxel Incoherent Motion Diffusion-Weighted Magnetic Resonance Imaging with Histopathological Correlation. PLoS One 2016;11:e0167720. [Crossref] [PubMed]
- Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806-17. [Crossref] [PubMed]
- Wang C, Zheng B, Chen Y, et al. Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs. Chin J Cancer Res 2013;25:63-70. [PubMed]
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25:1107-13. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Liu X, Qiu H, Zhang P, et al. Prognostic factors of primary gastrointestinal stromal tumors: a cohort study based on high-volume centers. Chin J Cancer Res 2018;30:61-71. [Crossref] [PubMed]
- Zhou Y, Hu W, Chen P, et al. Ki67 is a biological marker of malignant risk of gastrointestinal stromal tumors: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7911. [Crossref] [PubMed]
- Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 2011;37:890-6. [Crossref] [PubMed]
- Alghamdi HM, Amr SS, Shawarby MA, et al. Gastrointestinal stromal tumors. A clinicopathological study. Saudi Med J 2019;40:126-30. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Rizzo FM, Palmirotta R, Marzullo A, et al. Parallelism of DOG1 expression with recurrence risk in gastrointestinal stromal tumors bearing KIT or PDGFRA mutations. BMC Cancer 2016;16:87. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Liu Q, Wang Y, Kong L, et al. Study on Clinicopathological Features of Gastrointestinal Stromal Tumor and Relevant Prognostic Factors. Cell Biochem Biophys 2015;73:743-7. [Crossref] [PubMed]
- Liu X, Qiu H, Wu Z, et al. A Novel Pathological Prognostic Score (PPS) to Identify "Very High-Risk" Patients: a Multicenter Retrospective Analysis of 506 Patients with High Risk Gastrointestinal Stromal Tumor (GIST). J Gastrointest Surg 2018;22:2150-7. [Crossref] [PubMed]
- Apostolou KG, Schizas D, Vavouraki E, et al. Clinicopathological and Molecular Factors, Risk Factors, Treatment Outcomes and Risk of Recurrence in Mesenteric and Retroperitoneal Extragastrointestinal Stromal Tumors. Anticancer Res 2018;38:1903-9. [PubMed]
- Segales-Rojas P, Lino-Silva LS, Aguilar-Cruz E, et al. Association of ki67 Index with Recurrence in Gastrointestinal Stromal Tumors. J Gastrointest Cancer 2018;49:543-7. [Crossref] [PubMed]
- Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110-8. [Crossref] [PubMed]
- Wang Q, Huang ZP, Zhu Y, et al. Contribution of Interstitial Cells of Cajal to Gastrointestinal Stromal Tumor Risk. Med Sci Monit 2021;27:e929575. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Turkel Kucukmetin N, Cicek B, Saruc M, et al. Ki67 as a prognostic factor for long-term outcome following surgery in gastrointestinal stromal tumors. Eur J Gastroenterol Hepatol 2015;27:1276-80. [Crossref] [PubMed]
- Jiang L, Cao M, Hu J, et al. Expression of PIN1 in Gastrointestinal Stromal Tumours and its Clinical Significance. Anticancer Res 2016;36:1275-80. [PubMed]
- Seven G, Kochan K, Caglar E, et al. Evaluation of Ki67 Index in Endoscopic Ultrasound-Guided Fine Needle Aspiration Samples for the Assessment of Malignancy Risk in Gastric Gastrointestinal Stromal Tumors. Dig Dis 2021;39:407-14. [Crossref] [PubMed]
- Hashmi AA, Faraz M, Nauman Z, et al. Clinicopathologic features and prognostic grouping of gastrointestinal stromal tumors (GISTs) in Pakistani patients: an institutional perspective. BMC Res Notes 2018;11:457. [Crossref] [PubMed]



