
Clinical features and prognostic factors of esophageal signet ring cell carcinoma: construction and validation of a model based on the SEER database
Introduction
Worldwide, esophageal cancer is the sixth leading cause of cancer-related death and the seventh most common cancer, with about 604,100 new cases reported each year (1). Patients often present with distant metastasis, poor performance status, and have an unfavourable outcome even with aggressive interventions (2). According to global surveillance data, the 5-year age-standardized net survival ranged from 10% to 30% (3). Among various types of esophageal cancers, esophageal signet ring cell carcinoma (SRCC) is a particular kind of adenocarcinoma characterized by a significant proportion of tumor cells (usually >50%) showing an eccentric nucleus pushed to the peripheral region by abundant mucin (4). It is estimated that esophageal SRCC accounts for 2.6–5.0% of all esophageal cancers (5-7). Several studies indicated that SRCC was derived from tumor stem cells and featured by poor differentiation, rapid growth, diffuse infiltration, and high metastatic rate (8,9).
More than half of all SRCC cases originate in the stomach, followed by the colon, esophagus, rectum, lung, pancreas and so on (10). SRCCs in other organs are most frequently diagnosed at a metastatic stage with a low tumor grade, and have been associated with a poor prognosis (11,12). SRCCs of the esophagus were also reported to have statistically significant worse survival compared to those of squamous cell and adenocarcinomas (10). However, due to the relative rarity of this tumor type, the evidence so far has come mainly from case reports and single institution reports (6,13-16). The comprehensive understanding of SRCC in the esophagus is not well illustrated, particular for long-term survival and prognostic factors.
The Surveillance, Epidemiology, and End Results (SEER) database supported by the National Cancer Institute has been providing statistical information about tumors since 1975. It contains data on confirmed cancer cases across the United States (U.S.) and covers approximately a third of the U.S. population. The SEER database has become an ideal resource for studying relatively rare tumors and exploring their association with survival. Nomogram displays a simple chart based on a statistical prediction model that calculates the probability of clinical events by taking into account the prognostic weight of each factor. Our study aimed to determine prognostic factors and to establish nomograms to investigate overall survival (OS) and cancer-specific survival (CSS) of patients with esophageal SRCCs. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1238/rc).
Methods
Study population
Data between 1975 and 2017 were collected using SEER*Stat software (v 8.3.5). Patients who were pathologically diagnosed with esophageal SRCC were included using the following SEER variables: Site specific codes (C15.0-C15.5, C15.8, C15.9) and International Classification of Diseases for Oncology, third Edition (ICD-O-3) 8490/3. Our exclusion criteria included (I) patients whose information was obtained from autopsy and death certificates and (II) patients whose clinicopathological information was missing or incomplete. All included patients were randomly assigned to a training cohort and a validation cohort in a ratio of 6:4.
As the SEER database is publicly available, ethical approval is not required. We collected clinicopathological variables, including sex, age, race, insurance status, marital status, histology grade, primary site, American Joint Committee on Cancer (AJCC) stage, treatment status (for surgery, chemotherapy and radiation) and survival time. Tumors were classified in accordance with the 7th AJCC Tumor-Node-Metastasis (TNM) staging manual, which was published in 2010, and so the years allowed for diagnosis ranged from 2010 to 2015. OS and CSS were defined as the time from the date of diagnosis to the last follow-up or death due to all causes or esophageal SRCC, respectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Baseline characteristics were compared using the Chi-square test and the Fisher exact probability test. Log-rank test was used to analyze univariate factors. Factors with a P value <0.05 in univariate analysis were carried into a multivariate Cox proportional hazard regression analysis to obtain the hazard ratio (HR) and corresponding 95% confidential interval (CI). Independent prognostic variables, identified by univariate and multivariate analyses, were used for generating nomograms to predict the 2- and 5-year OS and CSS rates. We measured the performance of the nomograms through Harrell’s concordance index (C-index) and the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. A calibration plot was applied to measure nomogram-predicted probabilities relative to actual probabilities, using a 45-degree line as an optimal model. Furthermore, the C-indexes of the nomograms were also compared to that of the 7th TNM staging system. Statistical analysis was performed using SPSS 22.0 and R version 3.4.2 software (http://www.r-project.org). All P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
We collected data from 401 patients who were diagnosed with esophageal SRCC after applying strict inclusion criteria (Figure 1). Of those, 241 patients were randomly grouped into the training cohort, and the remaining patients (n=160) were assigned to the validation cohort. For the training set, approximately 87.6% of the patients were male (n=211) and the remaining 30 were female. Most patients were over 60 years of age (74.3%), white (92.9%), married (55.2%) and insured (84.6%). The most-common primary site was lower third of the esophagus (n=207, 85.9%), while stage IV (n=88, 36.5%) was the most-common AJCC stage. Among them, 71 (29.5%), 59 (24.5%) and 178 (73.9%) received surgery, radiotherapy and chemotherapy. Patients in the validation set were comparable to those in the training set with respect to all clinicopathological features (Table 1).
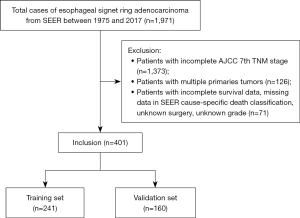
Table 1
| Variables | Total (n=401) | Training cohort (n=241) | Validation cohort (n=160) | P |
|---|---|---|---|---|
| Age (years), n (%) | 0.227 | |||
| <60 | 112 (27.9) | 62 (25.7) | 50 (31.3) | |
| ≥60 | 289 (72.1) | 179 (74.3) | 110 (68.7) | |
| Sex, n (%) | 0.340 | |||
| Female | 45 (11.2) | 30 (12.4) | 15 (9.4) | |
| Male | 356 (88.8) | 211 (87.6) | 145 (90.6) | |
| Race, n (%) | 0.368 | |||
| Black | 14 (3.5) | 9 (3.7) | 5 (3.1) | |
| White | 369 (92.0) | 224 (92.9) | 145 (90.6) | |
| Others/unknown | 18 (4.5) | 8 (3.4) | 10 (6.3) | |
| Marital status, n (%) | 0.09 | |||
| Married | 235 (58.6) | 133 (55.2) | 102 (63.8) | |
| Unmarried | 147 (36.7) | 93 (38.6) | 54 (33.8) | |
| Unknown | 19 (4.7) | 15 (6.2) | 4 (2.4) | |
| Insurance, n (%) | 0.867 | |||
| Insured | 340 (84.8) | 204 (84.6) | 136 (85.0) | |
| Any Medicaid | 43 (10.7) | 27 (11.2) | 16 (10.0) | |
| Uninsured/unknown | 18 (4.5) | 10 (4.2) | 8 (5.0) | |
| Grade, n (%) | 0.929 | |||
| I/II | 18 (4.5) | 11 (4.6) | 7 (4.4) | |
| III/IV | 383 (95.5) | 230 (95.4) | 153 (95.6) | |
| Primary site, n (%) | 0.581 | |||
| Upper/middle third | 21 (5.2) | 12 (5.0) | 9 (5.6) | |
| Lower third | 337 (84.0) | 207 (85.9) | 130 (81.2) | |
| Overlapping lesion | 19 (4.7) | 9 (3.7) | 10 (6.3) | |
| Esophagus, NOS | 24 (6.1) | 13 (5.4) | 11 (6.9) | |
| AJCC TNM stage (7th), n (%) | 0.850 | |||
| I | 39 (9.7) | 26 (10.8) | 13 (8.1) | |
| II | 76 (19.0) | 45 (18.7) | 31 (19.4) | |
| III | 137 (34.2) | 82 (34.0) | 55 (34.4) | |
| IV | 149 (37.1) | 88 (36.5) | 61 (38.1) | |
| Surgery, n (%) | 0.702 | |||
| No | 280 (69.8) | 170 (70.5) | 110 (68.8) | |
| Yes | 121 (30.2) | 71 (29.5) | 50 (31.2) | |
| Chemotherapy, n (%) | 0.265 | |||
| No/unknown | 113 (28.2) | 63 (26.1) | 50 (31.2) | |
| Yes | 288 (71.8) | 178 (73.9) | 110 (68.8) | |
| Radiation, n (%) | 0.689 | |||
| No/unknown | 300 (74.8) | 182 (75.5) | 118 (73.8) | |
| Yes | 101 (25.2) | 59 (24.5) | 42 (26.2) |
NOS, not otherwise specified; AJCC, American Joint Committee for Cancer; TNM, Tumor-Node-Metastasis.
Univariate and multivariate analyses of predictive factors on OS and CSS
Cox proportional hazards model was executed in the training set to investigate each variable’s ability to predict OS and CSS. Univariate analyses indicated that factors such as marital status, insurance, primary site, AJCC stage, surgery, chemotherapy and radiation therapy were associated with OS (Table 2). With regard to CSS analysis, age, insurance, primary site, AJCC stage, surgery, chemotherapy and radiation therapy were identified to be statistically significant predictive factors. Multivariate analyses confirmed that AJCC stage, surgery and chemotherapy were independent prognostic factors for OS and CSS.
Table 2
| Variables | Overall survival | Cancer-specific survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| Log rank χ2 | P | HR (95% CI) | P | Log rank χ2 | P | HR (95% CI) | P | ||||
| Sex | 1.829 | 0.176 | – | – | 0.720 | 0.396 | – | – | |||
| Female | |||||||||||
| Male | |||||||||||
| Age (years) | 9.170 | 0.057 | 0.454 | 10.401 | 0.034 | 0.327 | |||||
| <50 | Reference | Reference | |||||||||
| 50–59 | 0.734 (0.338–1.596) | 0.436 | 0.730 (0.338–1.575) | 0.422 | |||||||
| 60–69 | 0.573 (0.262–1.252) | 0.163 | 0.532 (0.244–1.159) | 0.112 | |||||||
| 70–79 | 0.682 (0.308–1.510) | 0.345 | 0.604 (0.271–1.343) | 0.216 | |||||||
| ≥80 | 0.528 (0.224–1.244) | 0.144 | 0.489 (0.205–1.167) | 0.107 | |||||||
| Race | 0.107 | 0.948 | – | – | 0.017 | 0.992 | – | – | |||
| Black | |||||||||||
| White | |||||||||||
| Others/unknown | |||||||||||
| Marital status | 6.199 | 0.045 | 0.525 | 5.191 | 0.075 | 0.472 | |||||
| Married | Reference | Reference | |||||||||
| Unmarried | 0.986 (0.717–1.357) | 0.933 | 0.971 (0.703–1.341) | 0.857 | |||||||
| Unknown | 1.446 (0.748–2.795) | 0.273 | 1.496 (0.752–2.976) | 0.251 | |||||||
| Insurance | 18.189 | 0.000 | 0.277 | 14.714 | 0.001 | 0.271 | |||||
| Insured | Reference | Reference | |||||||||
| Any Medicaid | 0.889 (0.543–1.455) | 0.639 | 0.796 (0.476–1.330) | 0.383 | |||||||
| Uninsured/unknown | 1.713 (0.822–3.573) | 0.151 | 1.609 (0.745–3.475) | 0.226 | |||||||
| Grade | 0.442 | 0.506 | – | – | 0.522 | 0.470 | – | – | |||
| I/II | |||||||||||
| III/IV | |||||||||||
| Primary site | 9.378 | 0.025 | 0.056 | 10.107 | 0.018 | 0.058 | |||||
| Upper/middle third | Reference | Reference | |||||||||
| Lower third | 0.745 (0.373–1.490) | 0.485 | 0.683 (0.342–1.366) | 0.281 | |||||||
| Overlapping lesion | 1.176 (0.451–3.069) | 0.741 | 1.144 (0.442–2.965) | 0.781 | |||||||
| Esophagus, NOS | 0.278 (0.097–0.801) | 0.018 | 0.261 (0.087–0.783) | 0.017 | |||||||
| AJCC TNM stage (7th) | 59.101 | 0.000 | 0.000 | 63.182 | 0.000 | 0.000 | |||||
| I | Reference | Reference | |||||||||
| II | 1.626 (0.822–3.216) | 0.163 | 1.555 (0.734–3.294) | 0.250 | |||||||
| III | 2.511 (1.291–4.881) | 0.007 | 2.625 (1.279–5.386) | 0.009 | |||||||
| IV | 4.523 (2.350–8.706) | 0.000 | 4.819 (2.375–9.779) | 0.000 | |||||||
| Surgery | 50.160 | 0.000 | 53.543 | 0.000 | |||||||
| No | Reference | Reference | |||||||||
| Yes | 0.339 (0.197–0.585) | 0.000 | 0.302 (0.166–0.548) | 0.000 | |||||||
| Chemotherapy | 16.260 | 0.000 | 11.220 | 0.001 | |||||||
| No/unknown | Reference | Reference | |||||||||
| Yes | 0.323 (0.215–0.486) | 0.000 | 0.346 (0.228–0.527) | 0.000 | |||||||
| Radiation | 28.489 | 0.000 | 28.568 | 0.000 | |||||||
| No/unknown | Reference | Reference | |||||||||
| Yes | 1.109 (0.623–1.974) | 0.724 | 1.137 (0.613–2.109) | 0.683 | |||||||
HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; AJCC, American Joint Committee for Cancer; TNM, Tumor-Node-Metastasis.
Establishment and validation the novel nomograms for OS and CSS
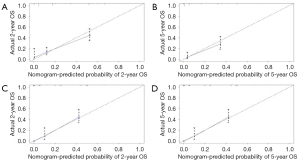
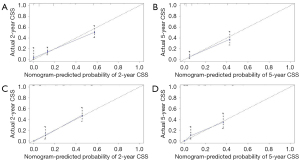
All independent prognostic factors which were determined by multivariate analyses were incorporated into the prognostic nomograms (Figure 2). Each risk factor corresponds to a specific score by drawing a vertical, upward line. The total score reflects the sum of the scores for each factor, and by drawing a vertical downward line, the predicted probabilities corresponding to the OS and CSS of 2 and 5 years can be obtained. The performance of the nomograms was then assessed using C-index, AUC values and calibration curves. In the training set, the C-index values of the nomogram and AJCC staging system for OS were 0.733 and 0.658, respectively. As for the validation group, the nomogram also improved discrimination of OS prediction compared to the AJCC staging system (0.785 vs. 0.675) (Table 3). In terms of CSS prediction, the predicted C-indexes of the nomogram and AJCC staging system were 0.737 and 0.666 in the training cohort, respectively. In addition, nomogram based on validation cohort also had significantly higher C-index value compared to the AJCC staging system (0.791 vs. 0.682), demonstrating more powerful CSS predictive efficiency (Table 3). Calibration curves for 2- and 5-year OS and CSS probabilities showed good consistency between nomograms predicting survival and actual observations of both training and validation groups (Figures 3,4).
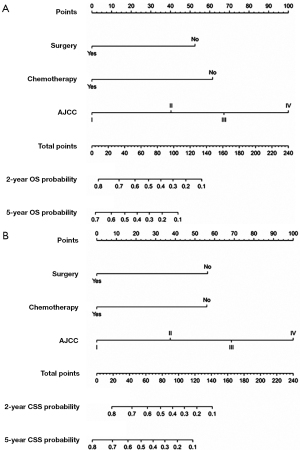
Table 3
| Survival | Training cohort | Internal validation cohort | |||
|---|---|---|---|---|---|
| C-index (95% CI) | P | C-index (95% CI) | P | ||
| Overall survival | <0.001 | <0.001 | |||
| Nomogram | 0.733 (0.696–0.770) | 0.785 (0.750–0.820) | |||
| 7th TNM stage | 0.658 (0.619–0.697) | 0.675 (0.628–0.722) | |||
| Cancer-specific survival | <0.001 | <0.001 | |||
| Nomogram | 0.737 (0.698–0.776) | 0.791 (0.756–0.826) | |||
| 7th TNM stage | 0.666 (0.625–0.707) | 0.682 (0.633–0.731) | |||
TNM, Tumor-Node-Metastasis; C-index, concordance index; CI, confidence interval.
Comparison of AUC values between the novel nomograms and 7th TNM staging system
Figures 5,6 showed the AUC values of nomograms used to predict the 2- and 5-year OS and CSS rates. The AUC values of the 2- and 5-year OS nomogram in the training cohort were 0.800 and 0.858, respectively, while the values of the 7th TNM staging system were 0.737 and 0.809, respectively (Table 4). As for the 2- and 5-year CSS prediction, the AUC values of the nomograms were higher than those of the 7th TNM staging system. Similar findings were demonstrated in the verification cohort.
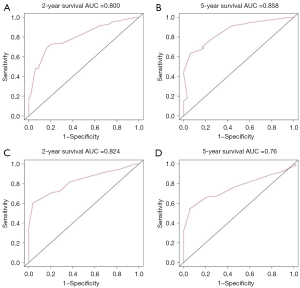
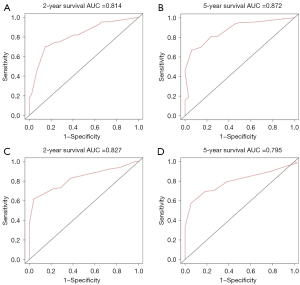
Table 4
| Survival | Training cohort | Internal validation cohort | |||
|---|---|---|---|---|---|
| 2-year survival | 5-year survival | 2-year survival | 5-year survival | ||
| Overall survival | |||||
| Nomogram | 0.800 | 0.858 | 0.824 | 0.760 | |
| 7th TNM stage | 0.737 | 0.809 | 0.737 | 0.713 | |
| Cancer-specific survival | |||||
| Nomogram | 0.814 | 0.872 | 0.827 | 0.795 | |
| 7th TNM stage | 0.751 | 0.845 | 0.730 | 0.700 | |
AUC, area under the curve; TNM, Tumor-Node-Metastasis.
Discussion
SRCC in primary non-gastric, colorectal sites is very rare, and most of the previous researches had small sample sizes or reported cases (5,7,13,14,16-18). Studies have shown that signet-ring cell (SRC) tumors are more commonly low and undifferentiated pathological grade, and the majority of them are advanced stage with distant metastasis (19-21). Our study found approximately 70% esophageal SRCC cases were diagnosed in stage III/IV, and more than 90% of cases had III/IV pathological grade. Our finding was in accordance with a recent study which reported that most gastroenteropancreatic SRCCs occurred in stage III/IV, and the grading distribution tended to be low differentiated or undifferentiated pathological grades at the time of diagnosis (22). So far, studies focusing on survival and prognosis of SRCC in the esophagus have been limited. Our study demonstrated that AJCC stage, chemotherapy and surgery were independent prognostic factors for OS and CSS. Furthermore, we constructed well-calibrated prognostic nomograms for predicting OS and CSS in patients with esophageal SRCCs. In both the training and the validation groups, better discriminative power of the nomograms was confirmed by the higher AUC values compared with the 7th AJCC staging system.
SRCC of the esophagus is infrequent. Most of what we know about SRCC is inferred from gastric cancers, which accounts for the vast majority of SRCC cases. Wu et al. demonstrated that the location of primary tumor might be an independent prognostic factor for CCS in patients with SRCCs (23). Another study showed that SRCC patients had the worst median OS in pancreas, together with SRCCs in the stomach and esophagus had the second worst OS among all the gastroenteropancreatic SRCCs, which might indicate potential different molecular characteristics (22). The novel nomograms in our study included several independent prognostic factors—AJCC stage, surgery and chemotherapy, among which AJCC stage had the highest discriminating power. In 2017, Chen and colleagues analyzed the clinicopathological parameters and prognosis of Chinese esophageal SRCC and found that the increase TNM stage was an independent risk factor for esophageal SRCC patients (13). In the same year, Wan et al. also reported similar findings that tumor invasion of adjacent organs, regional lymph node metastasis and distant metastasis were predictive factors of high disease-specific mortality in esophageal SRCCs (24). Consistent with these studies, our report demonstrated that tumor stage was significantly related to OS and CSS of esophageal SRCC patients. Thus, early detection and intervention may be critical for the long-term survival in patients with esophageal SRCCs.
Esophageal SRCC behaves differently from typical adenocarcinoma in its response to chemoradiotherapy and surgical regimens. Some studies have investigated the benefits of preoperative chemoradiotherapy for esophageal SRCC, but the results have been conflicting (15,17,25). Bekkar et al. showed that neoadjuvant radiochemotherapy was responsible for tumoral downstaging, reduced disease recurrence, and improved patient survival in locally advanced SRCC of the esophagogastric junction adenocarcinomas (17). A similar study by Chirieac et al. also found that patients diagnosed with adenocarcinoma of the esophageal or esophagogastric junction containing signet ring cells or mucus components benefited significantly from neoadjuvant radiochemotherapy and esophagectomy (15). On the other hand, Patel et al. demonstrated that esophageal SRCC did not respond well to neoadjuvant chemoradiotherapy, and could not benefit from preoperative therapy even if the tumors were downstaged (25). One possible reason for the discrepancy in these studies was that specific surgical, radiotherapy and chemotherapy techniques varied among studies. The length of some published studies has allowed patients enrolled later to benefit from better imaging techniques, which may also influence treatment choices and clinical outcomes. Additionally, the variations in the reporting of presence of SRCs also make it hard to make precise conclusions. According to our nomograms, chemotherapy was a significant prognostic factor, with a relatively high C-index among all predictive factors. Future subgroup analyses according to different chemotherapy regimens should be carried out to validate its role in esophageal SRCC.
In addition to chemotherapy, the present study showed that surgery was another independent prognostic factor for OS and CSS in esophageal SRCC patients. Esophagectomy was considered as the main method for the treatment of localized esophageal SRCC. Chen et al. found that the incidence of incomplete resections in SRC group was higher than that in the reference group (13), which was in line with previous studies (6,25). The authors suggested that the higher percentage of positive margins was not necessarily a sign that the tumor itself was more aggressive, but might be due to bias such as a longer delay in diagnosis. And this could also explain the lower survival in esophageal SRCC compared with its adenocarcinomas counterpart.
Competing risk nomograms based on the SEER database have been used in a variety of cancers, such as gastric cancer, liver cancer and lung cancer (26-28). Several studies indicated that compared to the AJCC staging system, nomograms can better estimate individual patient survival by integrating significant prognostic parameters (29,30). Our study is the first to develop competitive risk nomograms to predict OS and CSS for esophageal SRCC patients. In both the training and verification cohorts, nomograms were considered to have better discrimination with higher C-indexes and AUC values than the 7th AJCC staging system. The validity of the nomograms was also verified by the calibration curves. Moreover, during patient-clinician communication, our novel nomograms comprised of a few easily accessible clinical variables can help clinicians accurately estimate individual prognosis and thereby design appropriate treatment strategies for different patients.
There are several limitations in this study. First, our study was limited to retrospective data collection, which may lead to inevitable bias. The variables of chemotherapy and radiotherapy were classified as “yes” or “no/unknown” in the SEER dataset, we cannot accurately distinguish between “no treatment” and “unknown” if patients received treatment. Second, the sequence of treatment variables was not considered. Since tumor recurrence and progression were not recorded in the dataset, we had to use treatment as a baseline variable rather than a time-dependent covariable. In the absence of an exact time of treatment, we assume that it is determined and given at the time of diagnosis. This assumption is necessary to incorporate treatment information into the model. Third, although we use a large cohort to build the nomograms and verified in validation cohort, further external validation of more large queues is required to assess the accuracy of the predictive models. Despite these limitations, our study remains the largest population-based esophageal SRCC study and provides an instructive and effective model of esophageal SRCC prognosis. Additionally, our study confirmed the feasibility of nomogram in generating a numerical probability of survival in esophageal SRCC patients and provided a direction for future multicentre, large-scale cohort studies with adequate follow-up time.
Conclusions
AJCC stage, chemotherapy and surgery were independent prognostic factors affecting OS and CSS in patients with esophageal SRCC. The proposed three-factor nomogram can help clinicians accurately predict the prognosis of esophageal SRCC, thus contributing to individualized clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1238/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1238/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1238/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 2018;41:210-5. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Yendamuri S, Huang M, Malhotra U, et al. Prognostic implications of signet ring cell histology in esophageal adenocarcinoma. Cancer 2013;119:3156-61. [Crossref] [PubMed]
- Nafteux PR, Lerut TE, Villeneuve PJ, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg 2014;260:1023-9. [Crossref] [PubMed]
- Enlow JM, Denlinger CE, Stroud MR, et al. Adenocarcinoma of the esophagus with signet ring cell features portends a poor prognosis. Ann Thorac Surg 2013;96:1927-32. [Crossref] [PubMed]
- Gopalan V, Smith RA, Ho YH, et al. Signet-ring cell carcinoma of colorectum--current perspectives and molecular biology. Int J Colorectal Dis 2011;26:127-33. [Crossref] [PubMed]
- Hugen N, van de Velde CJH, de Wilt JHW, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Benesch MGK, Mathieson A. Epidemiology of Signet Ring Cell Adenocarcinomas. Cancers (Basel) 2020;12:1544. [Crossref] [PubMed]
- Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009;250:878-87. [Crossref] [PubMed]
- Hugen N, Verhoeven RH, Lemmens VE, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int J Cancer 2015;136:333-9. [Crossref] [PubMed]
- Chen L, Liu X, Gao L, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: A 10-year retrospective study in China. PLoS One 2017;12:e0176637. [Crossref] [PubMed]
- Maezato K, Nishimaki T, Oshiro M, et al. Signet-ring cell carcinoma of the esophagus associated with Barrett's epithelium: report of a case. Surg Today 2007;37:1096-101. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Correa AM, et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005;11:2229-36. [Crossref] [PubMed]
- Terada T. Signet-ring cell carcinoma of the esophagus in dermatomyositis: a case report with immunohistochemical study. J Gastrointest Cancer 2013;44:489-90. [Crossref] [PubMed]
- Bekkar S, Gronnier C, Messager M, et al. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg 2014;97:303-10. [Crossref] [PubMed]
- Honoré C, Goéré D, Messager M, et al. Risk factors of peritoneal recurrence in eso-gastric signet ring cell adenocarcinoma: results of a multicentre retrospective study. Eur J Surg Oncol 2013;39:235-41. [Crossref] [PubMed]
- Lu M, Yang Z, Feng Q, et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore) 2016;95:e4052. [Crossref] [PubMed]
- Nitsche U, Friess H, Agha A, et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res Clin Oncol 2016;142:2357-66. [Crossref] [PubMed]
- Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012;19:2814-21. [Crossref] [PubMed]
- Li H, Zong Z, Zhou T, et al. Trends of incidence and survival in patients with gastroenteropancreatic signet ring cell carcinoma: an analysis from the Surveillance, Epidemiology, and End Results program. J Gastrointest Oncol 2019;10:979-88. [Crossref] [PubMed]
- Wu SG, Chen XT, Zhang WW, et al. Survival in signet ring cell carcinoma varies based on primary tumor location: a Surveillance, Epidemiology, and End Results database analysis. Expert Rev Gastroenterol Hepatol 2018;12:209-14. [Crossref] [PubMed]
- Wan Z, Huang Z, Chen L. Survival predictors associated with signet ring cell carcinoma of the esophagus (SRCCE): A population-based retrospective cohort study. PLoS One 2017;12:e0181845. [Crossref] [PubMed]
- Patel VR, Hofstetter WL, Correa AM, et al. Signet ring cells in esophageal adenocarcinoma predict poor response to preoperative chemoradiation. Ann Thorac Surg 2014;98:1064-71. [Crossref] [PubMed]
- Yu C, Zhang Y. Development and validation of prognostic nomogram for young patients with gastric cancer. Ann Transl Med 2019;7:641. [Crossref] [PubMed]
- Ge D, Luo Z, Mao R, et al. Development and Validation of a Nomogram-Based Prognostic Evaluation Model for Sarcomatoid Hepatocellular Carcinoma. Adv Ther 2020;37:3185-205. [Crossref] [PubMed]
- Shang X, Yu H, Lin J, et al. A Novel Nomogram including AJCC Stages Could Better Predict Survival for NSCLC Patients Who Underwent Surgery: A Large Population-Based Study. J Oncol 2020;2020:7863984. [Crossref] [PubMed]
- Chen S, Lai Y, He Z, et al. Establishment and validation of a predictive nomogram model for non-small cell lung cancer patients with chronic hepatitis B viral infection. J Transl Med 2018;16:116. [Crossref] [PubMed]
- Roberto M, Botticelli A, Strigari L, et al. Prognosis of elderly gastric cancer patients after surgery: a nomogram to predict survival. Med Oncol 2018;35:111. [Crossref] [PubMed]


