
Risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer and its impact on mortality—a retrospective study
Introduction
The incidence of hepatitis B in China is high. Long-term hepatitis B viral infection and inflammation can promote liver fibrosis and cirrhosis, and eventually leads to liver cancer (1-3). Surgery is the main treatment method for patients with liver cancer, but the incidence of postoperative complications such as postoperative portal vein thrombosis is higher in these patients and is reportedly as high as 3.4% (4). Portal vein thrombosis can lead to cirrhosis or liver necrosis and can also lead to intestinal necrosis if concomitant with mesenteric vein thrombosis (5). Patients with mild portal thrombosis can be treated conservatively, while those with Yerdel grade III or IV often require surgery, which involves a high risk of death (4). It is clear that the treatment of portal thrombosis is very difficult, which can seriously affect the prognosis of the patients, and thus, preventing postoperative portal thrombosis in patients with hepatitis B liver cancer is crucial. Identifying the risk factors in these patients is key to preventing postoperative portal vein thrombosis.
A foreign study showed that advanced age and left hemihepatectomy are risk factors for portal thrombosis in patients with liver cancer (4). In Western countries, excessive alcohol consumption is the main cause of liver cancer, while long-term hepatitis B infection is the primary cause in China (6). Therefore, the risk factors for portal thrombosis in patients with liver cancer may be different in China from other countries. So further investigation is necessary. The present study aimed to explore the risk factors of postoperative portal thrombosis in hepatitis B liver cancer patients and its impact on the prognoses of these patients and to provide a basis for subsequent clinical interventions. We present the following article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1837/rc).
Methods
General information
We retrospectively included 663 patients with hepatitis B liver cancer admitted to the Second Affiliated Hospital of Anhui Medical University and Shenzhen University General Hospital from January 2017 to December 2021. The patients were divided into a portal thrombosis group (n=54) and a control group (n=609) according to whether they developed portal thrombosis after surgery.
The inclusion criteria were as follows: (I) patients with hepatitis B liver cancer (diagnosis basis: pathology); (II) those in whom the liver cancer could be surgically removed; (III) patients aged 18–75 years old; (IV) those who underwent liver cancer resection; and (V) patients with complete medical records. The exclusion criteria were as follows: (I) patients with contraindications to surgery; (II) those with extra-hepatic metastases; (III) patients with organ dysfunctions, such as liver or kidney dysfunction; (IV) those with myocardial infarction, stroke, and other severe cardiovascular diseases; (V) patients with other concomitant malignant tumors; (VI) patients with previous coagulation dysfunction diseases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (No. 20220175) and Shenzhen University General Hospital (No. 20220089). Informed consent was waived in this retrospective study.
Treatment methods
After admission, the patients underwent liver cancer resection within a limited period and were given symptomatic supportive treatment (including anti-infection, nutritional support, and maintenance of water-electrolyte balance) after surgery. Patients were routinely followed up at 3 months postoperatively with ultrasound or computed tomography (CT), and angiography or magnetic resonance imaging (MRI) was used to confirm the diagnosis in patients with suspected portal thrombosis.
Observation data
The observational data included age, sex, smoking, alcoholism, Child-Pugh liver function grading, D-dimer, surgical method (open surgery or minimally invasive), surgical range (semi-hepatic resection or lobectomy), maximum tumor diameter, diabetes mellitus, hypertension, postoperative abdominal infection, postoperative bleeding, and whether the spleen was removed. The Yerdel grading of portal thrombosis and prognosis (mortality and reoperation rate) were also studied.
Yerdel grading criteria for portal thrombosis
Grade I: portal vein obstruction is less than 50% of the portal vein lumen and does not extend to the superior mesenteric vein.
Grade II: portal vein obstruction is 50% to 100%, with or without extension to the superior mesenteric vein.
Grade III: the portal vein and proximal superior mesenteric vein are completely blocked, while the distal superior mesenteric vein is still unobstructed.
Grade IV: complete obstruction of the portal vein as well as the proximal and distal superior mesenteric veins. See Figure 1.
Statistical analysis
SPSS26.0 (IBM, Chicago, USA) was used for data analysis. The counting data for both groups were expressed by n (%) and the differences between the two groups were analyzed using the chi-square test. The measurement data of both groups were expressed by mean ± standard deviation and the differences between the two groups were analyzed by the independent sample t-test.
Binary multivariate logistics regression analysis was used to explore risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer (Forward LR). The diagnostic value of D-dimer for postoperative portal vein thrombosis in patients with hepatitis B liver cancer was analyzed by the Receiver operator characteristics (ROC) curve. P<0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
Analysis of the clinical and pathological features of patients in both groups
Compared with the control group, the proportion of D-dimer >8.74 mg/L in the portal thrombosis group was significantly increased (75.93% vs. 48.77%, P=0.000), the proportion of open surgery increased markedly (64.81% vs. 27.75%, P=0.000), and the proportion of the maximum tumor diameter >5 cm was notably increased (59.26% vs. 37.77%, P=0.002; Table 1).
Table 1
| Group | Portal thrombosis group (n=54) | Control group (n=609) | χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 2.752 | 0.097 | ||
| >65 | 30 (55.56%) | 267 (43.84%) | ||
| ≤65 | 24 (44.44%) | 342 (56.16%) | ||
| Gender | 0.011 | 0.917 | ||
| Male | 27 (50.00%) | 309 (50.74%) | ||
| Female | 27 (50.00%) | 300 (49.26%) | ||
| History of smoking | 1.283 | 0.257 | ||
| Yes | 11 (20.37%) | 89 (14.61%) | ||
| No | 43 (79.63%) | 520 (85.39%) | ||
| Alcohol | 0.778 | 0.378 | ||
| Yes | 9 (16.67%) | 76 (12.48%) | ||
| No | 45 (83.33%) | 533 (87.52%) | ||
| Diabetes | 0.924 | 0.337 | ||
| Yes | 9 (16.67%) | 74 (12.15%) | ||
| No | 45 (83.33%) | 535 (87.85%) | ||
| Hypertension | 0.064 | 0.801 | ||
| Yes | 5 (9.26%) | 63 (10.34%) | ||
| No | 49 (90.74%) | 546 (89.66%) | ||
| Child-Pugh liver function grading score >5 | 0.069 | 0.792 | ||
| Yes | 22 (40.74%) | 237 (38.92%) | ||
| No | 32 (59.26%) | 372 (61.08%) | ||
| D-dimer >8.74 mg/L | 14.639 | 0.000 | ||
| Yes | 41 (75.93%) | 297 (48.77%) | ||
| No | 13 (24.07%) | 312 (51.23%) | ||
| Surgical methods | 31.989 | 0.000 | ||
| Open surgery | 35 (64.81%) | 169 (27.75%) | ||
| Minimally invasive surgery | 19 (35.19%) | 440 (72.25%) | ||
| Scope of surgery | 2.314 | 0.128 | ||
| Hemihepatectomy | 22 (40.74%) | 187 (30.71%) | ||
| Lobectomy | 32 (59.26%) | 422 (69.29%) | ||
| Maximum tumor diameter >5 cm | 9.586 | 0.002 | ||
| Yes | 32 (59.26%) | 230 (37.77%) | ||
| No | 22 (40.74%) | 379 (62.23%) | ||
| Postoperative intra-abdominal infection | 2.465 | 0.116 | ||
| Yes | 3 (5.56%) | 13 (2.13%) | ||
| No | 51 (94.44%) | 596 (97.87%) | ||
| Postoperative bleeding | 1.528 | 0.216 | ||
| Yes | 1 (1.85%) | 3 (0.49%) | ||
| No | 53 (98.15%) | 606 (99.51%) | ||
| Combined splenectomy | 1.506 | 0.220 | ||
| Yes | 2 (3.70%) | 9 (1.48%) | ||
| No | 52 (96.30%) | 600 (98.52%) |
Diagnostic value of D-dimer for postoperative portal thrombosis in patients with hepatitis B liver cancer
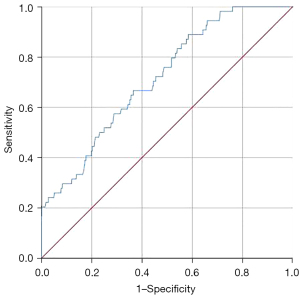
Compared with the control group, the level of D-dimer in patients with portal thrombosis was significantly increased (10.74±2.44 vs. 8.56±2.58 mg/L, P=0.000). The area under the ROC curve for the diagnostic value of D-dimer for postoperative portal vein thrombosis in patients with hepatitis B liver cancer was 0.716 (95% CI: 0.650–0.781, P=0.000; Figures 2,3).
Risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer
The multifactor logistics regression analysis showed that alcoholism, D-dimer >8.74 mg/L, open surgery, and a maximum tumor diameter >5 cm were risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer (P<0.05, Table 2).
Table 2
| Category | B | S.E. | Wald | P value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Alcohol | 1.095 | 0.452 | 5.882 | 0.015 | 2.991 (1.234–7.249) |
| D-dime >8.74 mg/L | 1.185 | 0.339 | 12.232 | 0.000 | 3.269 (1.683–6.349) |
| Surgical method (open surgery) | 1.906 | 0.345 | 30.477 | 0.000 | 6.726 (3.419–13.232) |
| The maximum tumor diameter >5 cm | 0.893 | 0.305 | 8.573 | 0.003 | 2.443 (1.344–4.442) |
| Constant | −5.505 | 1.308 | 17.705 | 0.000 | 0.004 |
B, intercept; S.E., Standard error.
Yerdel grading of portal vein thrombosis and prognosis of the patients
We found that 77.78% of patients had Grade I portal vein thrombosis, 18.52% of the patients had grade II portal vein thrombosis, 3.70% of patients had grade III portal vein thrombosis, and 0.00% of patients had grade IV portal vein thrombosis. Patients with Yerdel Grade I or II (96.30%) were cured after treatment, and two patients with Grade III (3.70%) died after surgery (Table 3).
Table 3
| Yerdel grade | n (%) | Prognosis |
|---|---|---|
| I | 42 (77.78) | All healed after anticoagulation |
| II | 10 (18.52) | Nine patients recovered after anticoagulation and one recovered after surgery |
| III | 2 (3.70) | Both cases died after surgery |
| IV | 0 (0.00) | – |
Discussion
Postoperative portal vein thrombosis is a common complication after hepatic resection, and most patients with portal vein thrombosis may have no obvious symptoms. However, if not treated in time, the mortality rate can be extremely high, especially when the portal vein thrombosis develops to Grade III or IV. Grade III or IV portal vein thrombosis can lead to refractory ascites, liver failure, and intestinal necrosis. Patients with grade III or IV portal vein thrombosis are treated with anticoagulation and operation, but the mortality rate is high even with aggressive surgical treatment (7,8). Therefore, preventing portal vein thrombosis is crucial, and the key to prevention lies in identifying the risk factors. Our study found that the incidence of portal vein thrombosis after surgery in patients with hepatitis B liver cancer was 8.14%, and alcoholism, D-dimer >8.74 mg/L, open surgery, and a maximum tumor diameter >5 cm were risk factors for postoperative portal thrombosis in patients with hepatitis B liver cancer (P<0.05). Also, patients with Yerdel grades I and II were cured after treatment, while two patients with Yerdel grade III died.
At present, the incidence of portal vein thrombosis after hepatic resection is mainly based on relevant foreign studies; the incidence rate is between 3% and 10% (4,8,9), although it has been reported to be as high as 20% (10). The fluctuating incidence of portal vein thrombosis after hepatic resection may be due to the absence of significant clinical symptoms in some mild patients and the omission of consultation. In terms of risk factors, right-sided hepatic resection, caudal lobectomy, splenectomy, and postoperative bile leakage are independent risk factors for portal vein thrombosis after hepatic resection (8). Advanced age and left hemisphere resection have also been shown to be risk factors for portal vein thrombosis in patients with liver cancer (4). Unlike previous studies, the present study showed an increased trend in portal thrombosis in elderly patients, but the difference was not statistically significant. In addition, our study also showed that splenectomy was not a risk factor for portal vein thrombosis after liver cancer surgery, mainly due to the small proportion of patients combined with splenectomy, which did not result in a statistically significant difference. Moreover, our study showed that alcoholism, D-dimer >8.74 mg/L, open surgery, and a maximum tumor diameter >5 cm were risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer (P<0.05).
Long-term alcoholism can lead to cirrhosis of the liver and vascular hardening, which can easily lead to thrombosis. There is fibrin in the blood, which is activated and hydrolyzed to produce a specific degradation product called "fibrin degradation product". D-dimer is the simplest fibrin degradation product. Increased D-dimer levels indicate that there is a high coagulation state and secondary hyperlysis of fibrinolysis in the body; so, thrombosis is easily induced when the D-dimer level is high (11-14). Some studies have also found that D-dimer has certain diagnostic values for portal vein thrombosis (15-17), which is consistent with our findings. Open surgery is traumatic and the risk of intraoperative injury to the portal vein is increased; thus, the risk of portal vein thrombosis after open surgery is increased (4). Finally, our study showed that a maximum tumor diameter >5 cm is also a risk factor for postoperative portal vein thrombosis. For large tumors, it is often necessary to remove multiple lobes or perform a semi-liver resection. In these cases, the surgical trauma is significant, the scope of resection is large, and some patients still require portal vein treatment. Ultimately, this will lead to an increased risk of postoperative portal vein thrombosis.
Our study showed that 77.78% of patients with Grade I portal vein thrombosis, 18.52% of patients with grade II portal vein thrombosis, 3.70% of patients with grade III portal vein thrombosis, and 0.00% of patients with grade IV portal vein thrombosis. Patients with Yerdel Grade I or II (96.30%) were cured after treatment, while two patients with Grade III (3.70%) died after surgery. Patients with grades I and II tended to have inconspicuous symptoms and most recovered with aggressive anticoagulation treatment (18-20). However, it is important to note that if patients with Yerdel grade I or II are not treated in time, it may develop into grade III or IV. For patients with grades III and IV, it is more dangerous, and untimely management can lead to liver failure, refractory ascites, intestinal necrosis, etc., which can in turn lead to death (21,22).
Limitations
This study had some limitations that should be noted. Firstly, this is a retrospective clinical study. In addition, we failed to explore the specific mechanisms of portal vein thrombosis after surgery in patients with hepatitis B liver cancer.
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Shenzhen University General Hospital (No. SUGH2018QD001); the Shenzhen Basic Research Programme (No. JCYJ20190808111610984); and the Youth Program of the National Natural Science Foundation of China (No. 81800586).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1837/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1837/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1837/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (No. 20220175) and Shenzhen University General Hospital (No. 20220089). Informed consent was waived in this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campbell K. Hepatitis B and the liver cancer endgame. Nature 2022;603:S64-5. [Crossref]
- Cao M, Fan J, Lu L, et al. Long term outcome of prevention of liver cancer by hepatitis B vaccine: Results from an RCT with 37 years. Cancer Lett 2022;536:215652. [Crossref] [PubMed]
- Kim BH, Lee D, Jung KW, et al. Cause of death and cause-specific mortality in primary liver cancer in Korea: A nationwide population-based study in hepatitis B virus endemic area. Clin Mol Hepatol 2022;28:242-53. [Crossref] [PubMed]
- Mori A, Arimoto A, Hamaguchi Y, et al. Risk Factors and Outcome of Portal Vein Thrombosis After Laparoscopic and Open Hepatectomy for Primary Liver Cancer: A Single-Center Experience. World J Surg 2020;44:3093-9. [Crossref] [PubMed]
- Wei Q, He Z, Wang K, et al. Prediction model based on blood urea nitrogen and the leukocyte count for intestinal necrosis in patients with portal vein system thrombosis: a retrospective study. Ann Transl Med 2020;8:326. [Crossref] [PubMed]
- Yang F, Ma L, Yang Y, et al. Contribution of Hepatitis B Virus Infection to the Aggressiveness of Primary Liver Cancer: A Clinical Epidemiological Study in Eastern China. Front Oncol 2019;9:370. [Crossref] [PubMed]
- Mori A, Iida T, Iwasaki J, et al. Portal vein reconstruction in adult living donor liver transplantation for patients with portal vein thrombosis in single center experience. J Hepatobiliary Pancreat Sci 2015;22:467-74. [Crossref] [PubMed]
- Kuboki S, Shimizu H, Ohtsuka M, et al. Incidence, risk factors, and management options for portal vein thrombosis after hepatectomy: a 14-year, single-center experience. Am J Surg 2015;210:878-85.e2. [Crossref] [PubMed]
- Yoshiya S, Shirabe K, Nakagawara H, et al. Portal vein thrombosis after hepatectomy. World J Surg 2014;38:1491-7. [Crossref] [PubMed]
- Takata H, Hirakata A, Ueda J, et al. Prediction of portal vein thrombosis after hepatectomy for hepatocellular carcinoma. Langenbecks Arch Surg 2021;406:781-9. [Crossref] [PubMed]
- Miyamoto K, Komatsu H, Okawa M, et al. D-dimer level significance for deep vein thrombosis screening in the third trimester: a retrospective study. BMC Pregnancy Childbirth 2022;22:21. [Crossref] [PubMed]
- Wang H, Lv B, Li W, et al. The Impact of D-Dimer on Postoperative Deep Vein Thrombosis in Patients with Thoracolumbar Fracture Caused by High-Energy Injuries. Clin Appl Thromb Hemost 2022;28:10760296211070009. [Crossref] [PubMed]
- Wang H, Lv B, Li W, et al. Diagnostic Performance of the Caprini Risk Assessment Model Combined With D-Dimer for Preoperative Deep Vein Thrombosis in Patients With Thoracolumbar Fractures Caused by High-Energy Injuries. World Neurosurg 2022;157:e410-6. [Crossref] [PubMed]
- Zhang J, Fang Y, Pang H, et al. Association of age-adjusted D-dimer with deep vein thrombosis risk in patients with spinal cord injury: a cross-sectional study. Spinal Cord 2022;60:90-8. [Crossref] [PubMed]
- Malaguarnera M, Latteri S, Bertino G, et al. D-dimer plasmatic levels as a marker for diagnosis and prognosis of hepatocellular carcinoma patients with portal vein thrombosis. Clin Exp Gastroenterol 2018;11:373-80. [Crossref] [PubMed]
- Dai J, Qi X, Li H, et al. Role of D-dimer in the Development of Portal Vein Thrombosis in Liver Cirrhosis: A Meta-analysis. Saudi J Gastroenterol 2015;21:165-74. [Crossref] [PubMed]
- Fei Y, Zong GQ, Chen J, et al. Evaluation of the Value of d-Dimer, P-Selectin, and Platelet Count for Prediction of Portal Vein Thrombosis After Devascularization. Clin Appl Thromb Hemost 2016;22:471-5. [Crossref] [PubMed]
- Galante A, De Gottardi A. Portal vein thrombosis: an overview of current treatment options. Acta Gastroenterol Belg 2021;84:327-32. [Crossref] [PubMed]
- Chun HS, Choe AR, Lee M, et al. Treatment of direct oral anticoagulants in patients with liver cirrhosis and portal vein thrombosis. Clin Mol Hepatol 2021;27:535-52. [Crossref] [PubMed]
- Caiano LM, Riva N, Carrier M, et al. Treatment of portal vein thrombosis: an updated narrative review. Minerva Med 2021;112:713-25. [PubMed]
- Yeo JW, Law MSN, Lim JCL, et al. Meta-analysis and systematic review: Prevalence, graft failure, mortality, and post-operative thrombosis in liver transplant recipients with pre-operative portal vein thrombosis. Clin Transplant 2022;36:e14520. [Crossref] [PubMed]
- Manatsathit W, Patel K, Enke T, et al. Increased Morbidity and Mortality of Patients with Non-cirrhotic Portal Vein Thrombosis After Abdominal and Pelvic Surgeries: a Study of the National Inpatient Sample 2002 to 2015. J Gastrointest Surg 2021;25:2026-34. [Crossref] [PubMed]
(English Language Editor: A. Kassem)




