
High expression of CD73 contributes to poor prognosis of clear-cell renal cell carcinoma by promoting cell proliferation and migration
Introduction
Renal cell carcinoma (RCC) is the second leading cause of death in urologic malignancy and accounts for 2% to 3% of all human malignancies (1). Histologically, approximately 80% of RCC is clear-cell renal carcinoma (ccRCC) (2,3). It’s reported that 25% to 30% of RCC patients present with metastatic disease at the time of diagnosis, and one-third of initially cured patients experience systemic progression (4,5). What’s worse, RCC is poorly response to radiotherapy and chemotherapy, the 5-year survival rate of metastatic RCC is only 10% (6,7). With remarkable progress of molecular targeted drug and PD-1/PD-L1 checkpoint blockade immunotherapy, it has significantly improved patients’ prognosis for those with metastatic RCC. However, it also brings severe side effects and drug tolerance (8). Therefore, clarifying the underlying molecular mechanisms of tumor progression and identifying new promising biomarkers are urgently needed to improve RCC prognosis.
CD73, also known as ecto-5’-nucleotidase, is a glycosyl phosphatidylinositol-anchored membrane protein encoded by the gene NT5E. One of the most important functions, CD73 is involved in the metabolic process of adenosine 5’-triphosphate (ATP). ATP is dephosphorylated to adenosine monophosphate (AMP) mediated by CD39 (ectonucleoside triphosphate dephosphohydrolase-1, ENTPD-1). CD73 then catalyzes the conversion of AMP to adenosine, which is a rate-limiting step. Being the key enzyme of the adenosine production process, CD73 plays important roles in various diseases. Functionally, CD73 has both enzymatic and non-enzymatic activities. On one hand, CD73 catalyzes the dephosphorylation of AMP to adenosine as an enzyme. On the other hand, CD73 is involved in the process of cell migration and invasion as an adhesion molecule.
The role of CD73 in different cancer cells has been also widely studied. It’s indicated that high expression of CD73 is associated with poor prognosis in various cancers, including melanoma (9), papillary thyroid carcinoma (10), hepatocellular carcinoma (11), triple-negative breast cancer (12), and colorectal cancer (13). For RCC, a recently study also showed that high CD73 expression portended significantly worse disease-free survival (DFS) and overall survival (OS), indicating that CD73 might be a potent therapeutic target for RCC patients (14). However, it remains unclear whether CD73 acts a role in cell proliferation and migration in patients with RCC.
Thus, in the present study, we detected the expression and evaluated the prognostic significance of CD73 in patients with pathologically confirmed ccRCC. The role of CD73 in cancer growth and progression in both in vitro and in vivo models were furtherly explored. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-544/rc).
Methods
Patients
A total of 72 patients who underwent nephrectomy and pathologically confirmed ccRCC between December 2012 and July 2015 in the fifth affiliated hospital of Sun Yat-sen University were retrospectively analyzed. Patient information and the paraffin specimens were collected. Additionally, 8 fresh pairs of ccRCC tissues and the matched normal tissues were immediately stored at −80 ℃ for further use. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (No. 2020#L235-1) and informed consent was taken from all individual participants.
Cell culture
Four human renal carcinoma cell lines (ACHN, 786-O, 769-P, Caki-1) and a proximal tubule epithelial cell line (HK2) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM or RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) at 37 ℃ with 5% CO2.
Lentiviral infection
Lentiviral construction of downregulated or overexpressed CD73 was designed and purchased from Shanghai Genechem Co. Knockdown or overexpression of CD73 in the stable transfectants were selected with puromycin (2 mg/mL) and verified by western blotting.
Immunohistochemistry (IHC) analysis
The paraffin specimens were stained according to procedures previously described (15). Staining for CD73 was underwent using a rabbit monoclonal anti-CD73 antibody (1:100, ab133582, Abcam) and goat anti-rabbit secondary antibody (BD5100, Bioworld). The score of CD73 expression was calculated by multiplying the staining intensity with the area of positive cells. For staining intensity, the score was quantified as 0 (negative), 1 (weak), 2 (medium) and 3 (intense). While for the area of positive cells, the score was quantified as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%). After multiplying, the final staining score was ranged from 0 to 12. And the scores of 0–4 and 5–12 were respectively represented as low and high expressions. The above staining scores were assessed by two independent pathologists blinded to the clinical data.
Western blotting
Tissues or cell lysates were harvested, and the protein concentrations were detected. Then the proteins were separated using 10% SDS-polyacrylamide gel electrophoresis, transferred onto polyvinylidend difluoride membranes, and blocked and incubated with primary antibodies. Finally, the membranes were incubated with the secondary antibody and visualized by enhanced chemiluminescence (SuperSignal Pierce Biotechnology). The antibodies for western blotting were as follows: CD73 (ab133582, Abcam), p-JNK (#4668, Cell Signaling technology), EGFR (ab52894, Abcam) and GAPDH (bs-0755R, Bioss).
MTT assay and colony formation assay
For MTT assay, cells were seeded into 96-well plates. During a 6-day culture periods, MTT reagent (0.5 mg/mL) was added to cell culture per day (20 µL/well). The MTT solution was removed 4 h later, and dimethyl sulfoxide (150 µL/well) was then added to dissolve the formazan sediment. The optical density at 490 nm was measured using a microplate absorbance reader.
For colony formation assay, cells were seeded into 6-well plates at a low density of 800 cells per well. The cells were then cultured for 10–12 d, and stained with a crystal violet solution to visualize the results.
Wound healing and migration assay
For wound healing assay, cells were seeded into 6-well plates. After cells were grown to confluence, wounds were created using a sterile plastic tip and photomicrographs were taken immediately and at 24 h.
For migration assay, cells were seeded into the upper chamber (Corning, USA) with serum-free medium. The lower chamber was filled with 10% FBS, and cells were fixed with methanol, stained with crystal violet, and photographed after incubation for 24–36 h.
Xenograft assay in nude mice
Animal experiments were performed under a project license (No. 2022#K318-1) granted by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University in compliance with guidelines of the Fifth Affiliated Hospital of Sun Yat-sen University for the care and use of animals. Cells were inoculated subcutaneously into the right back of 6-week-old BABL/c nude mice (n=5 for each group). Tumor growth was measured twice a week on the 7th day post-implantation, and tumor volume was calculated in two perpendicular diameters according to the formula: tumor volume (mm3) = (width2×length)/2. On the day 32nd or 35th, the mice were sacrificed and the tumor tissues were removed.
Statistical analysis
Statistical analyses were conducted using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Pearson’s chisquare test or Fisher’s exact probability test was performed for categorical variables. Kaplan-Meier test was performed for survival analysis, and cox proportional hazards model was used for multivariate analysis. A P value of less than 0.05 was considered statistically significant.
Results
CD73 is highly expressed in ccRCC
Firstly, we detected the expression of CD73 in ccRCC. Western blot results of 8 pairs of ccRCC tissues and matched normal tissues indicated that CD73 was remarkably upregulated in ccRCC tissues compared with that in the matched normal tissues (Figure 1A). Online databases were furtherly analyzed, mRNA expression of CD73 was extracted from the TCGA database (https://tcga-data.nci.nih.gov/tcga/) and GEO dataset (http://www.ncbi.nlm.nih.gov/geo/). As shown in Figure 1B, CD73 was significantly overexpressed in RCC samples from the TCGA and three GEO datasets (including GSE76207, GSE15641 and GSE68417).
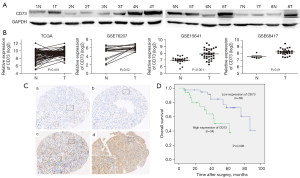
CD73 is associated with the prognosis of ccRCC
Furthermore, immunohistochemical expression of CD73 in 72 ccRCC patients was underwent. Membranous staining in cancer cells was observed, with a low expression of 38 patients and high expression of 34 patients according to the staining scores (Figure 1C). High expression of CD73 was significantly associated with higher Fuhrman grade (P=0.010) and higher pT status (P=0.031), while it was not related to age, gender, tumor side or tumor size (Table 1).
Table 1
| Characteristics | No of patients (n=72) | Expression of CD73, n (%) | P value | |
|---|---|---|---|---|
| Low (n=38) | High (n=34) | |||
| Age (years) | 0.988 | |||
| ≤63 | 53 | 28 (52.8) | 25 (47.2) | |
| >63 | 19 | 10 (52.6) | 9 (47.4) | |
| Gender | 0.574 | |||
| Male | 51 | 28 (54.9) | 23 (45.1) | |
| Female | 21 | 10 (47.6) | 11 (52.4) | |
| Tumor side | 0.370 | |||
| Left | 32 | 15 (46.9) | 17 (53.1) | |
| Right | 40 | 23 (57.5) | 17 (42.5) | |
| Tumor size (cm) | 0.282 | |||
| ≤4.0 | 44 | 21 (58.6) | 23 (41.4) | |
| >4.0 | 28 | 17 (44.4) | 11 (55.6) | |
| Fuhrman grade | 0.010 | |||
| I–II | 45 | 29 (64.4) | 16 (35.6) | |
| III–IV | 27 | 9 (33.3) | 18 (66.7) | |
| pT status | 0.031 | |||
| pT1–2 | 53 | 32 (60.4) | 21 (39.6) | |
| pT3–4 | 19 | 6 (31.6) | 13 (68.4) | |
ccRCC, clear-cell renal cell carcinoma.
To evaluate the role of CD73 in the prognosis of ccRCC, Kaplan-Meier (log-rank test) and multivariate analyses were conducted. Eligible patients were followed up for a median time of 39 month, ranged from 6–93 months. As shown in Figure 1D, patients with higher expression of CD73 had a worse overall survival time (P=0.006). On multivariate analyses, CD73 expression and Fuhrman grade were independently correlated with poor overall survival in patients with ccRCC (P=0.020 and P<0.001, respectively) (Table 2). These results indicated that CD73 might regulate the progression of ccRCC.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≤63 vs. >63 years) | 2.311 (0.958–5.574) | 0.062 | |||
| Gender (male vs. female) | 1.597 (0.681–3.744) | 0.282 | |||
| Tumor side (left vs. right) | 0.714 (0.309–1.653) | 0.432 | |||
| Tumor size (≤4.0 vs. >4.0 cm) | 0.850 (0.354–2.041) | 0.716 | |||
| Fuhrman grade (low vs. high) | 10.929 (3.975–30.051) | <0.001 | 10.716 (3.790–30.300) | <0.001 | |
| pT status (pT1–2 vs. pT3–4) | 1.006 (0.581–1.744) | 0.983 | |||
| CD73 expression (low vs. high) | 3.406 (1.359–8.541) | 0.009 | 3.146 (1.199–8.253) | 0.020 | |
ccRCC, clear-cell renal cell carcinoma; HR, hazard ratio; CI, confidence interval.
CD73 affects the proliferation and migration of ccRCC cells
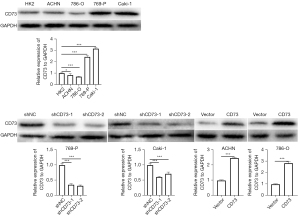
To furtherly explore the role of CD73 in ccRCC, we screened the expression of CD73 in four RCC cells (ACHN, 786-O, 769-P and Caki-1 cells) and normal proximal tubule epithelial cell line (HK2 cell). Our results showed that compared with HK2 cell, CD73 was downregulated in ACHN and 786-O cells, while upregulated in 769-P and Caki-1 cells (Figure 2). Therefore, in the subsequent experiments, we conducted CD73 knocked-down experiments in 769-P and Caki-1 cells and CD73 overexpressed experiments in ACHN and 786-O cells. The efficiency of knockdown or overexpress was confirmed by western blot assays (Figure 2).
Firstly, we observed the effect of CD73 on cell viability in vitro by using MTT assay and colony formation assay. The viability of 769-P and Caki-1 cells were significantly decreased after transfection with shCD73-1 or shCD73-2 (Figure 3). Conversely, overexpression of CD73 significantly enhanced the viability of ACHN and 786-O cells (Figure 4). Next, xenograft assays in nude mice were conducted to evaluate the effect of CD73 on tumor growth in vivo. As shown in Figures 3,4, tumors treated with knockdown of CD73 grew more slowly, while overexpression of CD73 promoted tumor growth.
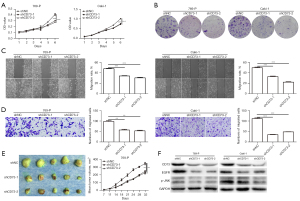
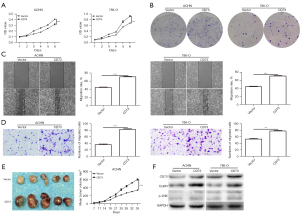
To determine whether CD73 could regulate the migration of ccRCC cells, wound healing and migration assay were performed. Interestingly, knockdown of CD73 inhibited the migration ability of ccRCC cells (Figure 3). Upon overexpression of CD73, migrative cells were significantly increased (Figure 4).
CD73 regulates the expression of EGFR and p-JNK
Several studies have showed that EGFR is involved in CD73-mediated tumor growth (16,17). Intriguingly, we found that knockdown of CD73 decreased the expression of EGFR, while overexpression of CD73 increased the expression of EGFR, which was consistent with previous research (Figures 3,4). According to our previous studies, the phosphorylation of JNK played an important role in adenosine signaling pathway (18,19). Therefore, we detected the effect of CD73 on the phosphorylation of JNK. As shown in Figure 3F and Figure 4F, CD73 knockdown decreased the phosphorylation of JNK, whereas CD73 overexpression promoted the phosphorylation of JNK.
CD73 acts both enzymatic and non-enzymatic function in ccRCC
CD73 has both enzymatic and non-enzymatic functions. To explore whether CD73 acted both enzymatic and non-enzymatic function in ccRCC, we examined the effect of APCP (a CD73 specific antagonist) on cell proliferation and migration using MTT and wound healing assay. As shown in Figure 5, APCP partially inhibited the effect of the CD73 overexpression on cell proliferation and migration, indicating that CD73 acts both enzymatic and non-enzymatic function in ccRCC.

Discussion
In the present study, we found that CD73 was upregulated in ccRCC and upregulation of CD73 was negatively correlated with prognosis in ccRCC. The mechanism was furtherly explored in vitro and in vivo, our data showed that suppression of CD73 inhibited cell proliferation and migration in ccRCC. Furthermore, CD73 acted both enzymatic and non-enzymatic functions in ccRCC.
Overexpression of CD73 has been demonstrated to be correlated with poor clinical outcomes in diverse cancers (9-13,20,21). In a cohort of 902 patients with gastric cancer, CD73 expression was more intensive in gastric cancer tissues and patients with high levels of CD73 had significantly poorer OS and DFS (22). Several studies also demonstrated that upregulation of CD73 was related with the resistance to antitumor therapies in cancer patients. For example, breast cancer patients with high expression of CD73 encountered poor response to trastuzumab (23). While silencing CD73 could enhance the chemosensitivity of NSCLC to cisplatin and potentiate cisplatin-induced anti-tumoral effects on NSCLC (24). Thus, CD73 may be a potential target and novel treatment approach in cancer.
So far, some studies have focused on the regulation of CD73 expression in cancer. One of the important factors is tumor microenvironment (TME). Hypoxia is a common feature of TME, especially in solid cancers. It has been well reported that hypoxia activates hypoxia-inducible factor 1-alpha (HIF-1α), which could bind to the promoter of NT5E gene and regulate the expression of CD73 (25). CD73 expression was then induced under hypoxic condition, leading to tumor progression (26,27). Epigenetic modification, such as DNA methylation, is also reported to be involved in regulation of CD73 expression in cancer. It’s reported that the methylation of NT5E gene was associated with clinical outcome in breast cancer and head and neck squamous cell carcinoma (28,29).
For renal cell carcinoma, there are few but growing studies have showed that CD73 was upregulated in RCC patients (14,30,31). The expression of CD73 was positively correlated with tumor stage (14,30) and Fuhrman grade (31), but negatively correlated with prognosis of patients (14,30,31). In the present cohort, a total of 72 ccRCC patients were analyzed and our results showed that high expression of CD73 was associated with Fuhrman grade and pT status, which was consistent with previous studies. Though the role of CD73 in prognosis and immunosuppression for RCC was explored, however these studies were all based on paraffin section, lacking verification of cell culture and animal experiment. In this study, we found that suppression of CD73 inhibited cell proliferation and migration in ccRCC both in vitro and in vivo. These findings furtherly indicated that CD73 was involved in the progression of ccRCC and might act as a potential target.
Epidermal growth factor receptor (EGFR), a member of the tyrosine kinase receptors family, acts a regulatory role in diverse fields of cell biology when activated through binding to epidermal growth factor. In addition to the physiological effect, EGFR activation is also found in the growth and progression of tumors (32,33). Recently, studies have showed a positive correlation between CD73 expression and EGFR in cancer. In hepatocellular carcinoma, high expression of EGFR was associated with strong CD73 expression (16). Similarly, EGFR expression was correlated positively to CD73 level in breast cancer (r=0.425, P<0.01) (17). In the present study, our data showed suppression of CD73 decreased the expression of EGFR, while overexpression of CD73 increased the expression of EGFR. These findings indicated that EGFR was involved in CD73-mediated tumor growth in ccRCC, and need to be furtherly explored in future studies.
As a nucleotidase, CD73 acts as an enzymatic role through catalyzing the conversion of AMP to adenosine. Cao et al. found that CD73 promoted the Warburg effect of human gastric cancer cells dependent on its enzyme activity (34). Aside from the enzymatic function, CD73 also plays a regulatory adhesion role between cells and extracellular matrix. Gao and his colleagues showed that CD73 promoted proliferation and migration of human cervical cancer cells independent of its enzyme activity (35). In the present study, we found that CD73 played both enzymatic and non-enzymatic functions in the regulation of cell proliferation and migration in ccRCC. The CD73 enzyme activity inhibitor (APCP) could partially inhibit the effect of the CD73 overexpression on cell proliferation and migration, which was consistent with previous studies in other cancers (16,34,36).
Conclusions
In conclusion, our data showed that CD73 was upregulated in ccRCC and high expression of CD73 was correlated with poor prognosis. In addition, we also showed that CD73 promoted the growth of ccRCC in vitro and in vivo. These findings suggest that CD73 may be a potential therapeutic target in ccRCC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-544/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-544/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-544/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-544/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (No. 2020#L235-1) and informed consent was taken from all individual participants. Animal experiments were performed under a project license (No. 2022#K318-1) granted by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University in compliance with guidelines of the Fifth Affiliated Hospital of Sun Yat-sen University for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022; Epub ahead of print. [Crossref]
- Bui TO, Dao VT, Nguyen VT, et al. Genomics of Clear-cell Renal Cell Carcinoma: A Systematic Review and Meta-analysis. Eur Urol 2022;81:349-61. [Crossref] [PubMed]
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-30. [Crossref] [PubMed]
- Lalani AA, McGregor BA, Albiges L, et al. Systemic Treatment of Metastatic Clear Cell Renal Cell Carcinoma in 2018: Current Paradigms, Use of Immunotherapy, and Future Directions. Eur Urol 2019;75:100-10. [Crossref] [PubMed]
- George DJ, Lee CH, Heng D. New approaches to first-line treatment of advanced renal cell carcinoma. Ther Adv Med Oncol 2021;13:17588359211034708. [Crossref] [PubMed]
- Govindarajan A, Castro DV, Zengin ZB, et al. Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions. Cancers (Basel) 2022;14:2049. [Crossref] [PubMed]
- Minguet J, Smith KH, Bramlage CP, et al. Targeted therapies for treatment of renal cell carcinoma: recent advances and future perspectives. Cancer Chemother Pharmacol 2015;76:219-33. [Crossref] [PubMed]
- Turiello R, Capone M, Giannarelli D, et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J Immunother Cancer 2020;8:e001689. [Crossref] [PubMed]
- Jeong YM, Cho H, Kim TM, et al. CD73 Overexpression Promotes Progression and Recurrence of Papillary Thyroid Carcinoma. Cancers (Basel) 2020;12:3042. [Crossref] [PubMed]
- Ma XL, Shen MN, Hu B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol 2019;12:37. [Crossref] [PubMed]
- Buisseret L, Pommey S, Allard B, et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol 2018;29:1056-62. [Crossref] [PubMed]
- Messaoudi N, Cousineau I, Arslanian E, et al. Prognostic value of CD73 expression in resected colorectal cancer liver metastasis. Oncoimmunology 2020;9:1746138. [Crossref] [PubMed]
- Tripathi A, Lin E, Xie W, et al. Prognostic significance and immune correlates of CD73 expression in renal cell carcinoma. J Immunother Cancer 2020;8:e001467. [Crossref] [PubMed]
- Zhou Y, Chu X, Deng F, et al. The adenosine A2b receptor promotes tumor progression of bladder urothelial carcinoma by enhancing MAPK signaling pathway. Oncotarget 2017;8:48755-68. [Crossref] [PubMed]
- Shali S, Yu J, Zhang X, et al. Ecto-5'-nucleotidase (CD73) is a potential target of hepatocellular carcinoma. J Cell Physiol 2019;234:10248-59. [Crossref] [PubMed]
- Zhi X, Wang Y, Yu J, et al. Potential prognostic biomarker CD73 regulates epidermal growth factor receptor expression in human breast cancer. IUBMB Life 2012;64:911-20. [Crossref] [PubMed]
- Yi Y, Zhou Y, Chu X, et al. Blockade of Adenosine A2b Receptor Reduces Tumor Growth and Migration in Renal Cell Carcinoma. J Cancer 2020;11:421-31. [Crossref] [PubMed]
- Zhou Y, Chu X, Yi Y, et al. MRS1754 inhibits proliferation and migration of bladder urothelial carcinoma by regulating mitogen-activated protein kinase pathway. J Cell Physiol 2019;234:11360-8. [Crossref] [PubMed]
- Chen YH, Lu HI, Lo CM, et al. CD73 Promotes Tumor Progression in Patients with Esophageal Squamous Cell Carcinoma. Cancers (Basel) 2021;13:3982. [Crossref] [PubMed]
- Gao ZW, Liu C, Yang L, et al. CD73 Severed as a Potential Prognostic Marker and Promote Lung Cancer Cells Migration via Enhancing EMT Progression. Front Genet 2021;12:728200. [Crossref] [PubMed]
- He X, Gu Y, Cao Y, et al. Impact of intratumoural CD73 expression on prognosis and therapeutic response in patients with gastric cancer. Eur J Cancer 2021;157:114-23. [Crossref] [PubMed]
- Turcotte M, Allard D, Mittal D, et al. CD73 Promotes Resistance to HER2/ErbB2 Antibody Therapy. Cancer Res 2017;77:5652-63. [Crossref] [PubMed]
- Baghbani E, Noorolyai S, Rahmani S, et al. Silencing tumor-intrinsic CD73 enhances the chemosensitivity of NSCLC and potentiates the anti-tumoral effects of cisplatin: An in vitro study. Biomed Pharmacother 2022;145:112370. [Crossref] [PubMed]
- Antonioli L, Yegutkin GG, Pacher P, et al. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2016;2:95-109. [Crossref] [PubMed]
- Sitkovsky MV, Hatfield S, Abbott R, et al. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2014;2:598-605. [Crossref] [PubMed]
- Samanta D, Park Y, Ni X, et al. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 2018;115:E1239-48. [Crossref] [PubMed]
- Lo Nigro C, Monteverde M, Lee S, et al. NT5E CpG island methylation is a favourable breast cancer biomarker. Br J Cancer 2012;107:75-83. [Crossref] [PubMed]
- Vogt TJ, Gevensleben H, Dietrich J, et al. Detailed analysis of adenosine A2a receptor (ADORA2A) and CD73 (5'-nucleotidase, ecto, NT5E) methylation and gene expression in head and neck squamous cell carcinoma patients. Oncoimmunology 2018;7:e1452579. [Crossref] [PubMed]
- Yu YI, Wang W, Song L, et al. Ecto-5'-nucleotidase expression is associated with the progression of renal cell carcinoma. Oncol Lett 2015;9:2485-94. [Crossref] [PubMed]
- Mei X, Shu J, Huang R, et al. Expression of VEGF, CD73 and their relationship with clinical pathology, microvessel density, and prognosis in renal cell carcinoma. Transl Androl Urol 2020;9:1366-73. [Crossref] [PubMed]
- London M, Gallo E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int 2020;44:1267-82. [Crossref] [PubMed]
- Uribe ML, Marrocco I, Yarden Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers (Basel) 2021;13:2748. [Crossref] [PubMed]
- Cao X, Zhu Z, Cao Y, et al. CD73 is a hypoxia-responsive gene and promotes the Warburg effect of human gastric cancer cells dependent on its enzyme activity. J Cancer 2021;12:6372-82. [Crossref] [PubMed]
- Gao ZW, Wang HP, Lin F, et al. CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer 2017;17:135. [Crossref] [PubMed]
- Wang L, Tang S, Wang Y, et al. Ecto-5'-nucleotidase (CD73) promotes tumor angiogenesis. Clin Exp Metastasis 2013;30:671-80. [Crossref] [PubMed]


